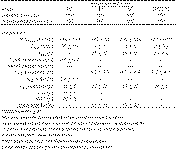8th Postgraduate Course for Training in Reproductive Medicine and Reproductive Biology
The Recombinant Follicle Stimulating Hormone : A New Alternative for Induction of Ovulation and Treatment of Polycystic Ovary Syndrome
D. Prasmusinto
Indonesia
Tutor
D. de Ziegler
Hopital de Nyon/ Department of Obstetrics and Gynecology, Geneva University Hospital
Introduction
In vitro fertilisation (IVF) is one of the several techniques being used for the treatment of infertility. In the early days it was used for treatment of infertility caused by inoperable tubal blockage. Through the years it became evident that a wider range of infertility patients can be treated by IVF.
In order to obtain ova for IVF, controlled ovarian hyperstimulation (COH) for ovulation induction is performed. COH results in more ova being available leading to better pregnancy rates and less side effects. Several substances are available to induce ovulation: clomiphen citrate, human menopausal gonadotrophin (hMG), human chorionic gonadotrophin (hCG), etc. Recently a recombinant follicle stimulation hormone (rFSH) has been developed which gives hope for even better results.
Follicle stimulating hormone (FSH) can be obtained by several techniques. One of them is by purifying urine from menopausal women and extract FSH and luteinizing hormone (LH). But LH may have a negative effect on ovulation induction. Therefore it is necessary to extract only FSH from the urine. Another technique to purify FSH is by using recombinant DNA technique.1 This technique has better results as it is not contaminated with other hormones and differences among batches will minimise.
In spite of infertility treatment, ovulation induction is also used to treat diseases such as polycystic ovary syndrome (PCO syndrome). PCO can be defined as LH-dependent hyperandrogenism. The syndrome has abnormalities in four endocrinologic active compartments :
- the ovaries,
- the adrenal gland,
- the peripheral fat tissue
- the hypothalamus-pituitary compartment.
One of the clinical features of PCO is amenorrhoea caused by anovulation.2
This report will summarise the use of rFSH for induction of ovulation, especially for IVF and treatment of PCO.
Follicular growth and ovarian function
The ovaries have two distinct functions, as a gamete and a sex hormone producer. During reproductive age, each month several follicles develop in the ovarian cortex. First the primordial follicle is formed, which develops into primary follicle, secondary follicle, tertiary follicle and eventually ovulation occurs. The remaining corpus luteum becomes atretic if no fertilisation takes place. The process of follicular growth is divided into two phases, follicular and luteal phase. 3
In its development the ovaries are influenced during the cycle by gonadotrophic hormones from the pituitary which stimulate preovulatory development and estradiol secretion. The pituitary is influenced by a hypothalamic peptide, which is a gonadotropin releasing hormone (GnRH).
During the follicular phase gonadotrophin hormones, mainly FSH, are needed for the development. This phase is divided into three steps :3,4
Follicular recruitment. It occurs during the first day of the cycle and affects maximal five follicles per ovary, 3-5 mm in diameter. At this time there is an elevation of plasma FSH level. FSH acts via a granulousa cell FSH receptors, is coupled with a cAMP mediated receptor and gives a signal to stimulate formation factors that modulate cell proliferation and differentiation. On the other hand, theca cells produce paracrine factors, including androgen, that are capable of influencing FSH action. It is evident that granulousa cell androgen receptors (AR), which mediate paracrine androgen action, are developmentally regulated by FSH. AR are found in preantral granulosa cells and in early intermediate antral follicles and disappear in the preovulatory follicle.
Follicular selection. This will result in the development of the future ovary. It concerns the follicle with the highest mitotic index and the largest diameter. At this time the FSH level decreases (through negative feed back caused by increasing estradiol) and signs of atresia appear in other follicles. Theca cells need luteinizing hormone (LH) to produce androgen as an estrogen precursor in FSH stimulated granulosa cells. FSH also stimulates granulosa cells to produce factors to up-regulate LH stimulated thecal P450 c 17 a mRNA expression and synthesis androgen. Androgen itself has two different regulatory roles in follicle development. In low concentrations, it can stimulate aromatase activity via AR. But in high concentrations, intense 5-a reductase activity will change androgen to the form that can not be aromatised. This androgen microenviroment will stop expression of AR and set the follicle on the path to atresia.
Follicular dominance. The selected follicle will exert and others will become atretic. The dominant follicle is rich on estrogen microenviroment and expresses most FSH receptors.
In early menstrual cycle, gonadal steroid levels are low. With the demise of the corpus luteum FSH level will increase and lead to follicle recruitment. Through estrogen produced by the follicle negative feedback will decrease pituitary FSH secretion and increase LH. LH receptors, induced by FSH, appear in granulosa cells, and through LH stimulation progesterone is produced. With sufficient estrogen stimulation, LH-surge is triggered and ovulation occurs. During the luteal phase, estrogen levels decrease in the beginning but will rise again in the mid-luteal phase. Estrogen is produced by the corpus luteum. Progesterone will increase after ovulation. Both estrogen and progesterone levels will stay high until the corpus luteum becomes atretic.3
Recombinant DNA
Recombinant DNA technology enables to improve a wide range of diagnostic and therapeutic areas. It can be used to identify, isolate, clone and produce specific proteins. It is possible to identify mutation, to diagnose affected or carrier states for hereditary diseases, to perform in situ hybridisation, to map the human genes on the chromosome, to isolate and alter genes, to transfer genes from one organism to another and to perform in vivo structure-function analyses. Another clinical advantage is the production of large quantities of specific protein products such as hormones, vaccines and other biologic agents of pharmacological importance.1
The basic concept of molecular biology is that DNA makes RNA and RNA makes protein.5 Before starting with recombinant DNA technology, individual genes have to be isolated from the background host genomic DNA. These genes must contain coding DNA sequences (exons) to produce proteins, a leader DNA sequence to promote transcription, noncoding DNA material (introns) which will be eliminated in the process of forming of RNA, and nucleotide sequences which are the terminal end of a gene and turn off the transcription process.5
Gene isolation needs to fragment DNA molecules that comprise 46 human chromosomes by using a bacterial enzyme named restriction enzyme (endonuclease). This bacteria will cleave DNA in specific sequences of DNA. A piece of human DNA cleave with a specific protease can be fused with a piece of bacterial DNA which cleaves with the same enzyme.1
Subsequent process is to duplicate the gene and its host cell (cloning). Through cloning a new cell is created that produces human protein and protein from the host cell gene (expression). To insert a strange DNA to host cell a vector is needed. A vector is a piece of DNA which can develop independently, for example a combination of foreign (human) and plasmid (bacteria) or phage (viral). The host cell and the recombinant plasmid are placed in a special nutrient mixture, designed for optimal cell viability and protein production.1
Purifying the crude protein is accomplished by a sequence of chromatographic steps to separate the desired protein from the crude mixture. Then the protein is mixed with stabilisers or buffer, specific for the protein product.1
Recombinant FSH
In recombinant gondotrophin, isolation of the specific gene of the hormone has been done. The FSH a, LH a , and hCG a subunits are identical. To isolate the a subunit from gondotrophin, placental tissue and DNA are homogenised and isolated by extraction. A DNA library is prepared by ligating size-fractionated placental DNA with phaga l and the hCG a gene is isolated. Cellular human pituitary RNA from 5 human pituitaries is used to isolate FSH b subunit and l cDNA library is prepared for the isolation of the FSH b gene.1
Both of the subunit genes are inserted into a vector with mammalian gene promoter (the a gene is directed from the simian virus-40 promoter, whereas the b gene is transcribed from the human or mouse metallothionein II A promoter), linked to gene encoding selectable markers, and then into the genome of a chinese hamster ovary (CHO) cell line.6
Recombinant FSH is identical to pituitary or urinary FSH in aminoacid sequence, glycosilation site, receptor binding capacity and in vitro biologic activity, whereas recombinant and natural carbohydrate structures are similar.
After purifying with immunochromatography of specific anti FSH monoclonal antibodies, the recombinant FSH preparation contains an average of 10.000 IU FSH/ mg protein.6
Pharmacokinetics of rFSH
In vitro and in vivo studies of rFSH and FSH from natural sources show very similar results. Le Cottonec et al7 studied the pharmacokinetics of rFSH (Gonal-F) and uFSH (Metrodin) after a single intravenous injection in 12 pituitary down-regulated healthy women. They found that the mean concentration time profiles after injection of 15 IU of uFSH and rFSH were almost similar and the mean profile after 300 IU of rFSH was twice that of the 150 IU dose. Drug exposure was similar for both products, and for rFSH it would increase in direct proportion to the dose. The initial (distribution) half life of both preparations was 2 hours and the true terminal (elimination) time was 17 hours. Approximately 20 % of the administered FSH and 10 % of the recombinant FSH was excreted in the urine. Renal clearance of uFSH was 0.1 L/h and it was lower for rFSH (0.07 L/h). Volume distributions in steady state were similar for both preparations (11L).
Le Cottonec et al8 also studied the single dose pharmacokinetics of rFSH, administered intravenous (IV), intramuscular (IM) or subcutaneous (SC) and its pharmacokinetic steady state after multiple SC dosing. After a single IV administration, drug exposure was 309 IU/l, 235 IU/l after SC and 177 IU/l after IM administration. The maximum increase of FSH serum concentration was 35 IU/l for IV and 3 IU/l for IM and SC. However, the maximal concentration was reached earlier with SC administration (16 hours) compared to IM route (25 hours). This situation was associated with the longer absorption half life observed with the IM route (8 hours) than with the SC route (5 hours). The terminal half life for IV was 18 hours, and for IM and SC routes 37 hours, but a great interindividual variability was observed in IM and SC routes (70 %). After repeated SC administration, terminal half life was 24 hours with smaller interindividual variability (30 %) and comparable with the one observed after single IV administration. The estimated total clearance was 0.6L/h and renal clearance was 0.06L/h. The initial volume of distribution was 4L, which corresponded well to the serum volume. The volume of distribution at steady state was 11L and the mean residence time was 20 hours (Table 1).
Porchet et al9 found good correlation between maximal inhibin levels and maximal total follicular volumes, and between increasing maximum estradiol serum level and maximal total follicular volume after administering 150 IU/l SC of rFSH for 7 days in down-regulated healthy volunteers despite complete LH supression. Total follicular volume was starting to increase 4 to 5 days after initiating rFSH when FSH serum concentration had already reached steady state. Inhibin, secreted by human granulous cells could be used as index of follicular development. The increase of inhibin appeared on day 3 after FSH administration indicating follicular development. Estradiol increased more slowly than inhibin and was associated with total follicular volume. The estradiol estimated average equilibration half life was approximately 4 days and at least 4 days were required between initiation of FSH therapy and estradiol increase.
Shoham et al10 reported data on dose-related increase of serum FSH after multiple dose of rFSH. Two hypogonadotrophic women received 75 IU rFSH/day IM for 7 days, then 150 IU rFSH/d for subsequent 7 days and 225 IU rFSH/d for the last 7 days. For every rFSH dose applied, steady state levels of FSH concentration were reached in 3-5 days.
Maximal effect of a given dose of rFSH administrated daily can not be observed until 3 or 4 days after repeated administration. It is advisable waiting for an efficacy effect for at least 4 days before increasing the dose.10
Table 1. Pharmacokinetics of rFSH8
Controlled ovarian hyperstimulation
Controlled ovarian hyperstimulation (COH) is widely used in in-vitro fertilisation, gamete intrafallopian transfer, intrauterine insemination and other assisted reproductive technologies. During a normal cycle follicle development is divided into 3 steps, at the end being only one follicle dominant. The purpose of COH is to develop more follicles. This process can be done by changing the FSH level.
Previously human menopausal gonadotropin (hMG) was used for COH. Human menopausal gonadotropin contains 75IU FSH and 75IU LH, purified from menopausal women's urine. By using hMG more follicles can be developed. But there is a premature surge of LH, which causes premature luteinisation as a response of increasing estrogen concentration. Another negative effect is ovarian hyperstimulation syndrome (OHSS) causing luteinisation of multiple follicles which can be lethal, especially in WHO group II amenorrhoea. this could be a result of an excess in LH factor which will stimulate theca cells to produce androgen as an estrogen precursor. In the beginning it was thought that this problem can be solved by adding GnRH agonists. But on the other hand it was found that there is no difference in the incidence of OHSS, midluteal ovarian size, and serum concentration of progesterone between hMG treatment and hMG plus GnRH agonist (leuprolid). To decrease theLH factor, the urine is purified in order to get pure FSH (Metrodin). Now it is has been shown that rFSH only contains pure FSH.12
Controlled ovarian hyperstimulation by using rFSH
Dovrey et al13 reported a successful pregnancy after stimulation with rFSH (Org 32489). Recombinant FSH was administered in conjunction with a GnRH agonist (buserelin) to obtain the oocytes in a woman with inoperable tubal damage. The function of the ovaries was still normal as judged by serial measurements of serum FSH, LH, estradiol and progesterone. Seventy five IU rFSH per day were administerd on days 3-11. Buserelin was given one day before the first FSH injection. Serum LH gradually declined from 3.8 IU/l on cycle day 1 to 0.9 IU/l on cycle day 11. After several days, serum FSH concentration was comparable to that measured on cycle day 1 (3-5 IU/l). A two fold increase of serum inhibin was noted and serum estradiol concentration increased rapidly from 36 pg/ml on cycle day 1 up to 2640 pg/ml on cycle day 12, when six pre ovulatory follicles (diameter 15-23 mm) were observed by transvaginal ultrasound. Then hCG (10.000 IU, Pergnyl Organon) was administered and 36 hours later 9 mature oocytes were retrieved. In total four oocytes were fertilised and there 4-cell embryos were transferred into the uterus . During the first week after hCG, an initial rise of serum estradiol and progesterone was apparent. The pregnancy progressed normal and ended with a vaginal delivery at 39.5 weeks.
Germond et al14 also reported a successful in-vitro fertilisation and embryo transfer after treatment with recombinant human FSH. A 32-year old woman with primary tubal infertility (bilateral tubal occlusion) was treated with rFSH (Gonal-F, Serono) subcutaneously to stimulate multiple follicular development and perform IVF/ET.
Buserelin was given in the midluteal phase and on day 2 after menstruation, daily treatment with 225 IU rFSH was started. On day 14 after hCG injection eight follicles were retrieved and four oocytes were recovered and fertilised in vitro. Twenty-eight days after the embryo transfer an intrauterine twin pregnancy was confirmed. Another report, by Schoot et al15, refers to a woman with isolated gonadotropin deficiency. The isolated gonadotropin deficiency occurs as part of a number of syndromes where the disorder lies within the GnRH pulse generator rather than being a failure of the pituitary gland to produce gonadotropin. In this case, GnRH neurones were absent in the hypothalamus and the olfactory bulb.16 This woman was suffering from primary amenorrhoea due to isolated congenital gonadotropin deficiency. Serum level for FSH was 1.2 IU/l and 0.37 IU/l for LH. She refrained from estrogen replacement therapy ten days prior to the study. 75 IU/d rFSH (Organon 32489) was given IM for seven days and subsequently increased to 150 IU/d for two weeks. One ovarian follicle (diameter 14 mm) was found on day 13 and medication was discontinued. On day 19, multiple follicles ( n=6, 12-18 mm) were observed. Meanwhile, after giving FSH for three weeks, endometrial thickness was observed by ultrasonography on alternate days and no sign of endometrial development was seen. In this study, it was found that follicle development coincided with increasing serum FSH concentration. Serum LH concentration was around 0.09 and 0.38 IU/l.
Figure 1. Serum FSH & LH level in a patient with isolated gonadotropin deficiency after rFSH15
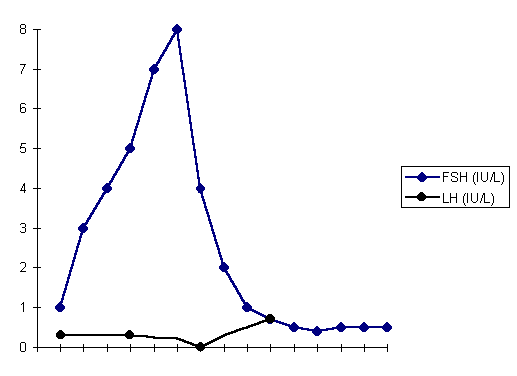
Serum estradiol level showed a gradual increase to a maximum on day 15, the day of maximum follicle size (236 pmol/L). Serum progesterone showed no elevation following hCG administration (Fig. 1 & 2).
Figure 2. Serum estradiol concentration of a patient with isolated gonadotropin deficiency after rFSH15
serum
This study suggested that FSH alone can induce growth of preovulatory follicles, even in the absence of normal estradiol levels. LH is needed for adequate androgen biosynthesis as a substrate for aromatase activity. In the study before, estrogen was still produced when LH levels were low (0.9 IU/l) whereas in the following study, LH levels were very low and could not stimulate estrogen. It was suggested that LH is needed although in a low concentration to stimulate estrogen. But it was assumed there was an internal factor that influenced estrogen stimulation besides LH. On the other hand, high LH levels can induce premature luteinizing and OHSS. It seems that the response of theca cells is individually different.
This supports the two cells, two gonadotropin theory. It is stated that there is a subdivision and compartmentalisation of steroid hormone synthesis activity in the developing follicle. Aromatisation of androgens takes place in granulosa cells (to produce estrogen). This process needs FSH stimulation via a specific receptor. The granulosa cells do not have enzymes involved in the early steroidogenic pathway and need androgen as substrate for aromatisation. Androgen is synthesised by LH stimulation of theca cells which have many LH receptors. LH stimulates theca cells to produce androgen and it transfers to granulosa cells for FSH-stimulated aromatisation into estrogen.
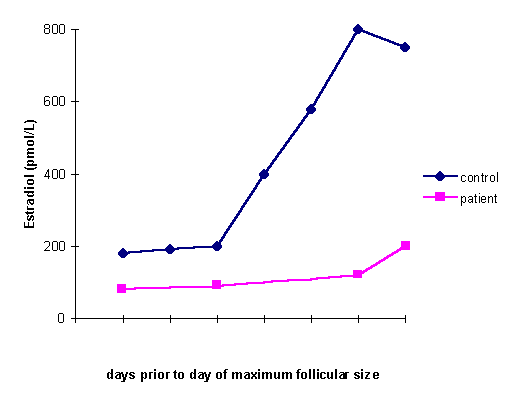
The Recombinant human FSH study group17 compared the efficacy and the safety of rFSH with uFSH for stimulating follicular development in women undergoing IVF-ET. They designed a multicenter, prospective, randomised, open, parallel group, clinical study. They found that there are no differences between rFSH and uFSH for follicular development in terms of number of oocytes, number of patients with at least one fertilised oocyte and a number of cleavage embryos (Table 2).
Out JH et18 al studied ongoing IVF pregnancy rates (PR) after treatment with rFSH (Puregon) compared to urinary gonadotropin (uFSH). A meta analysis including 25 IVF centers was performed. PR was measured 12 weeks after embryo transfer. Mean number of fresh embryos transferred was almost the same between rFSH and uFSH. The ongoing PR was higher in rFSH group (22.9 % vs 17.9%) which was statistically significant. If the cryoprogram was included, the difference between the treatments increased to 6.4%.
Table 2. Follicular development, ovum pick-up and IVF result17
Table 3. Number of pregnancies, deliveries, and PRs17
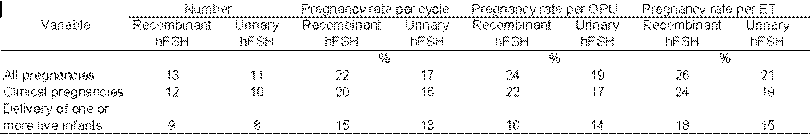
In the author’s opinion rFSH lead to better quality oocytes and embryos. Total oocytes retrieved and embryos obtained after rFSH treatment resulted in a surplus in embryos.The recombinant human FSH study group17 did not find significant differences between rFSH and uFSH in follicular development, ovum pick up (OPU) and IVF results (Table 3).
Strowitzki et al19 studied ovarian stimulation in women undergoing in-vitro fertilisation and embryo transfer using recombinant human follicle stimulating hormone (Gonal-F) in non down-regulated cycles. The women were divided into two groups, receiving 225 IU and 300 IU rFSH, respectively. A mean number of 6.26 and 5.88 oocytes was collected. The number of transferred embryos was 2.4 and 2.2. A clinical pregnancy rate of 23.8 % per transfer was achieved.
Wit regard to these studies, it seems that rFSH is as safe and effective as uFSH, or even better, in stimulating ovarian follicular development.
Polycystic ovary syndrome (PCO)
Association between amenorrhoea and bilateral PCO was first reported by Stein-Leventhal in 1935.20 PCO is defined as hyperandrogenism, chronic anovulation excluding secondary causes such as neoplasm, hyperprolactinemia and adult onset of adrenal hyperplasia. Disregulation of p450c17, the enzyme that forms androgen in adrenal glands and ovaries, can be the underlying mechanism of hyperandrogenism in PCO. LH has an important role in stimulating androgens. High intraovarian androgen concentration can delay follicle maturation. Estradiol level is still in follicular phase concentration, but estron is higher because of peripheral aromatisation of androstenedion and thereby reversing the E1:E2 ratio.2
FSH levls are not increased, which may be caused by the negative feedback from chronic elevated estrogen levels and normal follicular inhibin. Because GnRH stimulation is increased, LH concentration is high causing a change in the LH:FSH ratio.
PCO can cause infertility and anovulatory menstrual cycles. Treatment for PCO in the early 1960 as reported by Stein-Leventhal was antiestrogen and exogenous gonadotropin, besides ovarian wedge resection which was.20 If no response to antiestrogens occured, clomiphene citrate (CC) was given for induction of ovulation. But still several cases were resistant to CC (20-25 %) and ovarian wedge resection could lead to pelvic adhesion complications.
In the last 30 years, gonadotropin is used as a treatment for PCO and in case of resistance to CC. Frank and Hamilton-Farley21 defined CC-resistance if after giving three successive cycles of CC ovulation was not achieved (as confirmed by ultrasound and endocrine criteria) although the dose had been increased or if the woman after at least 6 ovulatory cycles and no other obvious infertility factors had not conceived. Besides for CC-resistant women, gonadotropin is also recommended for women having intolerable side effects with antiestrogens and for those who had two or more miscarriages after CC treatment.
Follicle Stimulating Hormone for PCO
The results after hMG treatment for PCO are not encouraging enough. Wang and Gemzeel 22 reported that from 724 women with PCO treated with hMG, ovulation rate was high (76 % - 95%) but PR was only 28 %. Premature luteinisation might be one explanation for the low PR rate. Frank and Hamilton-Farley21 found the following results: 46 % of patients were conceiving, 34 % multiple pregnancies, 4.6% hyperstimulated cycles (severe) and 23 % spontaneous miscarriage.
Purified preparations of urinary derived gonadotropin (hMG 3:1 [Organon] & Metrodin [Serono]) appeared to be ideal for treatment of PCO with LH elevation. The main advantage using uFSH after hMG for induction of ovulation was elimination of the majority of LH from the exogenous gonadotropin preparation. Less exogenous LH administered would improve the abnormal basal LH:FSH ratio found in many women with PCO syndrome. uFSH might decrease circulating concentrations of LH and alter the LH : FSH ratio favourably, but premature luteinisation, multiple follicle development and pregnancy rates appeared similar to those reported for hMG.23 Although Siebel et al24 reported that the prevalence of multiple follicles and hyperstimulation was less than with hMG treatment but this was not a prospective, randomized, comparative study.
Bennink et al12 compared rFSH with uFSH in women with CC-resistance, normogonadotropic, and chronic anovulation (WHO group II). This was a randomised, assessor blind, prospective and multicenter study. It included 172 patient, age 18-39 years with normal serum levels of FSH, prolactin, and TSH in the early follicular phase. A low dose step-up FSH regimen was used starting with 75 IU/d IM for up to 14 days and a weekly increment of half an ampoule given thereafter if needed until the threshold base for follicular development was achieved. The cumulative ovulation rates after three treatment cycles were 95 % and 96 % for rFSH and uFSH groups respectively. In total 69.5 % of the rFSH treated cycles were ovulatory compared to 66.7% of the uFSH treatment cycles. There was no difference in the mean number of follicles of > 15 mm and > 18 mm on the day hCG was administered. But mean number of follicles with a diameter of > 12 mm was significantly higher in the rFSH group compared with the uFSH group. Meanwhile median serum estradiol concentration was significantly higher in the rFSH group. (1,400 vs 1,000 pmol/l). Serum FSH level was significantly lower in the rFSH group (6.2 IU/l vs 7.1 IU/l) on the day of hCG administered. A lower total dose was found in rFSH group (750 vs 1,350 IU) which was statistically significant. The cumulative ongoing pregnancy rate after three cycles was 27% in the rFSH group and 24 % in the uFSH group, which was not statistically significant. OHSS was found in 7.6% of the women treated with rFSH and 4.6% the uFSH group. But only one woman (from rFSH group) was admitted to the hospital. Multiple gestation was found in both groups. These differences between the groups were not statistically significant. Differences in the isohormone profile, the pharmacological formulation, contamination of uFSH with possible FSH-inhibitory activity and small differences in the oligosaccharide structure might explain the increased effectiveness in rFSH compared to uFSH.
Polycystic ovarian syndrome and in vitro fertilisation
PCO patients receiving IVF program, using gonadotropin or gonadotropin and GnRH agonists, have a better PR and live birth rate compared to normo-ovulatory women. Dale et al24 reported in PCO patients that follicle aspiration resulted in 18.8 oocytes per cycle with a cleavage rate of 68% and a PR of 33.3% per embryo transfer and the accumulated pregnancy rate after transfer of cryopreserved was36.6 %.
Mac Dougal et al26 compared PCO patients to a control group. They found a significantly higher serum estradiol level on the day of hCG administration (5940 vs 4370 nmol), more follicles developed and more oocytes were produced in the PCO group, despite PCO patients receiving significantly less hMG. But fertilisation rate was reduced in the PCO patient. There were no significant differences in cleavage rates and PR/ET was 25.4% in the PCO group and 23% in the control. Multiple pregnancies were higher in PCO groups.
In these studies a high incidence of OHSS (10.5% - 46.7%) was seen in the PCO group, probably caused by the LH factor.
Trials in PCO patients undergoing IVF receiving rFSH treatment is seldom reported. Van Dessel et al27 in 1991 reported the first successful pregnancy after induction of ovulation with rFSH in PCO. A PCO woman, 27 years old, with infertility was participating in this study. Her LH serum was high, with a LH/FSH ratio 5.8. She was CC-resistant and had already used hMG-GnRH agonist but no pregnancy was achieved. 75 IU rFSH IM/d was administered on day 3 to 13 and one dominant follicle was found (18 mm). Ovulation was induced with hCG. Serum estradiol maximum on 18 mm follicles was 660 pmol/ l while serum progesterone concentration was 28 nmol/l after 8 days following hCG administration. No luteal support was provided. Pregnancy progressed without problem and a healthy 4600 g boy was born.
This case report showed the usefulness of rFSH in PCO. rFSH may not contain LH, but high LH concentration is associated with low conception and high miscarriage rates. It is assumed that in this case report, patient LH concentration was normal because we know gonadotropin preparation with low LH content have failed to show consistently improved results when used for PCO patients and not all of PCO women exhibit elevated LH concentration.
Conclusion
Recombinant FSH is a new alternative for induction of ovulation for in vitro fertilisation technique. By using this regimen, physicians can get good quality ova which may result in even better pregnancy rates. The advantages of rFSH are better isohormone profile, better pharmacokinetic formulations that will minimise differences between batches, no contaminating protein and small differences in the oligosaccharide structure.
It is thought that rFSH has also a better result in treating PCO syndrome, especially in patients that are clompihene citrate-resistant or not respond to gonadotropin and GnRH agonist regimen. But not many studies have been done so far to confirm this theory. Also, endogenous LH concentration influence the result of rFSH-treatment in PCO.
Acknowledgement
I would like to thank Prof. Campana, Chairman of the Department of Gynecology and Obstetrics of the Medical Faculty of the University of Geneva, Switzerland for a valuable chance that he has given me to study this topic. I also thank Dr. D. De Ziegler from the Department of Obstetrics and Gynecology, Hospital of Nyon, Switzerland for his attention and tutorial when I was doing this literature research.
References
- Shoham Z, Insler V. Recombinant technique and gonadotropin production : new era in reproductive medicine. Fertil Steril 1996; 66 : 187-201.
- Hershlag A, Peterson CM. Endocrin disorder. In : Berek JS, Adashi EY, Hillard PA (editors). Novak’s gynecology. 12th edition. Maryland : William & Wilkins, 1996 ; 837-45.
- Palter SF, Olive DL. Reproductive physiology. In : Berek JS, Adashi EY, Hillard PA (editors). Novak’s gynecology. 12th edition. Maryland : William & Wilkins, 1996; 149-69.
- Hillier SG. Roles of follicle stimulating hormone and luteinizing homone in controlled ovarian hyperstimulation. Human Reprod, 1996 ; 3 Suppl : 113-21
- Simpson AJ, Walker T, Terry R. An introduction to recombinant DNA technology. Parasitology, 1986 ; 92 Suppl : S7-14
- Mannaerts B, De Leeuw R, Geelen J, et al. Comparative in vtro and in vivo studies on the biological characteristic of recombinant follicle stimulating hormone. Endocrinology, 1991; 129 : 2632-30.
- Le Cottonec, Porchet H, Beltrami V, et al. Clinical pharmacology of recombinant follicle stimulating hormone (rFSH). I. comparative pharmacokinetics with urinary human follicle stimulating hormone. Fertil Steril, 1994 ; 61 : 669-78.
- Le Cottonec, Porchet H, Beltrami V, et al. Clinical pharmacology of recombinant follicle stimulating hormone (rFSH). II. Single doses and steady state pharmacokinetics. Fertil Steril, 1994 ; 61 : 679-86.
- Porchet H, Le Cottonec, Loumaye E. Clinical pharmacology of recombinant follicle stimulating hormone . III. Pharmacokinetic-pharmacodynamic modelling after repeated subcutaneous administration. Fertil Steril 1994; 61 : 687-95
- Shoham Z, Mannaerts B, Insler V, Bennink H. Induction of follicular growth using recombinant human follicle stimulating hormone in two volunteer women with hypogonadotropic-hypogonadism. Fertil Steril, 1993 ; 59 : 738-42.
- Rafael ZB, Levy T, Schoemarker J. Pharmacokinetics of follicle stimulating hormone : clinical significance. Fertil Steril, 1995 ; 63 : 689 - 700.
- Bennink C, Fauser B, Out H. Recombinant follicle stimulating hormone (FSH; Puregon) is more efficient than urynary FSH (Metrodin) in women with clomiphene citrate-resistant, normogonadotropic, chronic anovulation : a prospective, multicenter, assessor blind, randomised, clinical trial. Fertil Steril, 1998 ; 69 : 19-25
- Devroey P, Mannaerts B, Smitz J, Bennink C, Steirteghem AV. First established pregnancy and bith after ovarian stimulation with recombinant follicle stimulating hormone (Org 32489). Human Reprod, 1993 ; 8 : 863-65
- Germond M, Dessole S, Senn A, Loumaye E, Howles C, Beltrami V. Successful in vitro fertilisation and embryo transfer after treatment with recombinant human FSH. Lancet, 1992 ; 339 : 1170.
- Schoot D, Bennink C, Mannaerts B, Lamberts S, Bouchard P, Fauser B. Human recombinant follicle stimulating hormone induces growth of preovulatory follicles without concomitant increase in androgen and estrogen biosynthesis in a woman with isolated gonadotropin deficiency. J. Clin. Endocrinol Metab, 1992 ; 74 : 1471-73
- Rebar R. The puberty. In : Berek JS, Adashi EY, Hillard PA (editors). Novak’s gynecology. 12th edition. Maryland : William & Wilkins, 1996 ; 788-9.
- Recombinant human FSH study group. Clinical assessment of recombinant human follicle-stimulating hormone in stimulating ovarian follicular development befor in vitro fertilisation. Fertil Steril, 1995; 63 : 77-86.
- Out H, Driessen S, Mannaerts B, Bennink C. Recombinant follicle stimulating hormone (follitropin beta, Puregon*) yields higher pregnancy rates in in vitro fertilisation than urinary gonadotropin. Fertil Steril, 1997 ; 68 : 138-42.
- Strowitzki T, Kentenich H, Kiesel L, Neulen J, Bilger W. Ovarian stimulation in women undergoing in-vitro fertilisation and embryo transfer using recombinant human follicle stimulating hormone (Gonal-F) in non-down-regulated cycles. Human Reprod, 1995 ; 10 : 3097-3101.
- Stein IF, Leventhal ML. Amenorrhoea associated with bilateral poycystic ovaries. Am J Obstet Gynecol 1935 ; 29 : 181-91.
- Franks S, Hamilton-Farley D. Ovulation induction : Gonadotropin. In : Adashi E, Rock J, Rosenwaks Z (editors). Reproductive endocrinology, surgery, and technology. Philadelphia : Lippincott-Raven Publisher, 1996 : 1208-23.
- Wang CF, Gemzell C. The use of human gonadotropins for induction of ovulation in women with polycystic ovairan disease. Fertil Steril, 1980 ; 33 : 479-86
- Kamvara MM, Seibel MM, Berger MJ, Thompson I, Taylor ML. Reversal of persistent anovulation in polycystic ovarian disease by administration of chronic low-dose follicle stimulating hormone. Fertil Steril, 1982 ; 37 : 520-23.
- Seibel MM, McArdle C, Smith D, Taymor ML. Ovulation induction in polycystic ovarian syndrome with urinary follicle stimulating hormone or human menopausal gonadotropin. Fertil Steril, 1985 ; 43 : 703-8
- Dale PO, Tanbo T, Åbyholm T. In vitro fertilisation in infertile women with polycystic ovarian syndrome. Human Reprod, 1991;6: 238-41.
- MacDougall M, Tan SL, Balen A, Jacobs HS. A controlled study comparing patients with and without polycystic ovaries undergoing in vitro fertilisation. Human Reprod, 1993 ; 8 : 233-37.
- Van Dessel H, Donderwinkel P, Bennink C, Fauser B. Case report first established pregnancy and birth after induction of ovulation with recombinant human FSH in polycystic ovarian syndrome. Human Reprod, 1994 ; 9 : 55-6.