8th Postgraduate Course for Training in Reproductive Medicine and Reproductive Biology
Second Trimester Maternal Serum Screening Programmes for the Detection of Down's Syndrome
Arben Paralloi
Albania
Tutor
Dr. Celia De Lozier
Division of Medical Genetics
Geneva University Hospital
Introduction
Trisomy 21, or Down’s Syndrome, is the most common serious autosomal chromosome aberration in which affected individuals survive beyond infancy. The severity of the syndrome includes mental deficiency, congenital cardiac malformation, immune system disorders, gastro-intestinal malformation and slow physical development. After the age of 35, almost all affected individuals exhibit brain lesions similar to those observed in individuals with Alzheimer's disease. The overall risk of having a newborn with Down's syndrome is 0.13%.
It has been estimated costs to detect each case of fetal Down's syndrome is much lower compared with the average cost of health care, education and residential costs for such an individual. Also, taking into consideration the shock such a baby provides to his parents it is clear how important it is to have early prenatal diagnosis. At present there is no screening test available which is capable of specifically detecting parental predisposition for offspring with Down's syndrome. The established methods of detection are therefore based upon testing the fetus, either directly by invasive techniques or indirectly based upon indices of relative fetal development. Amniocentesis is an invasive technique in which a sample of the amniotic fluid is removed from the uterus. Fetal cells suspended in the fluid can then be cultured and examined for genetic disorders. This method is associated with approximately 0.5% - 1.0% increase in the risk of spontaneous abortion.
Chorionic villus sampling (CVS) is another invasive procedure performed in the first trimester, where a small piece of chorionic villus for chromosomal analysis is withdrawn. CVS results approximately in a 1.5% increased risk of spontaneous fetal losses through 28 weeks of gestation (1).
The risk associated with invasive diagnostic procedures and the costs of analysis preclude the adoption of methods for mass screening of pregnant women.
Maternal serum screening is a non-invasive method. The objective of this screening is to reduce the proportion of women who have to undergo invasive diagnostic testing and, at the same time, increasing the proportion of affected fetuses detected. It relies upon the measurement of multiple biochemical markers (AFP, Hcg, Ue3, free beta-Hcg) and calculation of the risk factor based upon the parameters measured and the mother's age at delivery. By this means, women at high risk of giving birth to a Down's syndrome child can be identified and offered confirmatory testing using a suitable invasive technique. Screening procedures vary from country to country. Controversy exists about the number of the markers used and which combination yields the highest efficacy. The result of the screening is a risk estimate. Parental counseling is essential to insure informed consent for further investigation and for any termination of pregnancy that may result. The decisions made based on the results of screening procedures are affected by cultural and moral standards as well as practical considerations.
Several factors influence the accuracy of screening tests, including gestation dating method, maternal weight, number of fetuses etc.
Principles of antenatal screening for Down syndrome
The risk of conceiving a fetus with Down's syndrome is significantly related to maternal age (3,4). The risk increases almost exponentially with age. For example, a woman at 35 years at 15-20 weeks has a 1 in 270 risk of gestation of carrying a Down's syndrome fetus (table 1). However, the risk of this woman bearing a live-born baby with Down's syndrome is 1 in 385, because it has been demonstrated that about 30% of fetuses with the syndrome are spontaneously aborted between mid-pregnancy and term.
Table 1. Risk of conceiving a fetus with Down's syndrome by maternal age.
|
Maternal Age (years) |
Risks |
|
20 |
1 : 1528 |
|
25 |
1: 1351 |
|
30 |
1 : 909 |
|
35 |
1 : 385 |
|
40 |
1 : 112 |
|
45 |
1 : 25 |
The first screening programs for Down's syndrome relied upon amniocentesis offered only to women of 35 years of age or older thus being a high risk population screening approach.
However, using this approach only about 20% -30% of all Down's syndrome pregnancies could be detected and then only if there was a 100% uptake rate, because the majority of these babies are born to women under 35 years of age. So despite the higher risk of Down's syndrome in the babies of older mothers, about 80% of Down's syndrome babies are born to young women. By using maternal age of 35 years or greater as the criteria for prenatal screening, most of the cases that occur in women less than 35 years old remain undetected. Also, because their age-related risks were lower than the risk of a subsequently pregnancy loss caused by amniocentesis.
Maternal serum screening is a non invasive method. It relies upon the measurement of multiple biochemical markers.
The principal biological maternal serum markers are:- Alpha-fetoprotein (AFP)
- Human chorionic gonadotropin (hCG)
- Free beta-hCG
- Unconjugated estriol (uE3)
- The detection rate which represents the sensitivity of the method, is defined as the number of Down syndrome cases correctly identified by the screening method.
- The false-positive rate can be used to assess the specificity of the test, because specificity is defined as 100% - the false positive rate.
- The particular methodology is the expression of the values of the markers in MoM(Multiple of the Median).This particularity implies that every laboratory has to determine its own medians by measuring the individual concentrations in a certain community of normal pregnant women.
- A third point is the importance of gestational dating by the aid of ultrasound examination (biparietal diameter) since serum markers vary with gestational age and therefore each measured value must be standardized against the expected median value for normal pregnancies at the same gestational age.
- The risk of having fetal Down's syndrome is calculated by multiplying the determined risk deriving from the maternal age by a multiplying factor (f) obtained from the curves of the distribution of the serum concentration observed in normal and affected pregnancies.
- Neural tube defects ( NTD ).
- Anencephaly, spina bifida, encephalocele
- Abdominal wall defects (AWD)
- Omphalocele, gastroschisis
- Other chromosomal anomalies such as Trisomy 18 (Edwards syndrome) (61)
MShCG level at 15 weeks is thought to be a predictor for preeclampsia and other complications of pregnancy(38)
The advent of maternal serum markers for Down syndrome screening
AFP
AFP is a glycoprotein with a molecular weight of 68000 daltons produced by the fetal yolk sac and fetal liver. Fetal plasma concentration increases to a maximum (approximately 3.0-4.0 g/L) between 13-14 weeks of gestation. Maternal serum levels peak at about 30 weeks (about 250 mg/L). After birth, maternal and infant AFP rapidly decline. In 1984 Merkatz and co-workers reported that maternal serum AFP in the second trimester from pregnancies affected with fetal trisomy 21 was lower than compared to normal pregnancies. It was also demonstrated that the decrease in MSAFP was independent of maternal age, making prenatal screening for fetal Down’s syndrome possible in women less than 35 years old. This association of decreased levels of MSAFP with an increased risk of Down syndrome led to the introduction of risk screening in which a woman's AFP level and age were used to estimate her odds of having an affected child (3).The report of Merkatz and co-workers was confirmed by other studies (6). The levels of MSAFP in Down's syndrome pregnancies are about 72% of the normal values for weeks 14-21.
It is obvious, by examining the distribution of MSAFP levels found in Down's syndrome versus unaffected pregnancies, that no level of MSAFP will cleanly separate affected from unaffected pregnancies. Because of the extensive overlap of the two distributions, selection of a cut-off corresponding to any reasonable detection rate will inevitably result in false-positives cases. The selection of a cut-off point therefore involves a compromise between the detection rate and the false-positive rate, as it is true for screening based on maternal age alone. A cut-off point selected so that 5% of women of all ages would have amniocentesis (about 0.5 MoM) would allow a detection rate of about 20%. Thus, the use of a fixed MSAFP cut-off could be about as effective as maternal age alone in detecting Down's syndrome fetuses in terms of the number of Down's syndrome fetuses detected per amniocentesis performed. However, an individual woman's risk of carrying a Down's syndrome fetus will vary at any given MSAFP MoM with this approach because the background risk based on her age will vary. For example, a 34-year-old woman with an MSAFP MoM of 0.5 would have a second trimester risk of about 1/148, but a 22-year-old woman with the same MoM would have a second trimester risk of about 1/450. A policy using a fixed screening cut-off point will recommend amniocentesis for some women having a relatively low risk.
Combining maternal age and MSAFP as a screening test for Down's syndrome
The most common approach to establish individualized MSAFP screening cut-off points in younger women uses the rationale that an individual's risk based on age and MSAFP level combined should be as great or greater than that of a woman at age 35, namely 1/385 at term. Whatever the individual pregnant woman's a priori risk is (estimated at the basis of her age alone), that risk will increase if her MSAFP value is relatively low and will decrease if the MSAFP value is relatively high. Hence, to estimate the risk for a given age and AFP level it is only necessary to multiply the age-specific odds ratio (Down's syndrome:unaffected pregnancy) by a factor known as ‘likelihood ratio’. The likelihood ratio is the proportion of Down's syndrome pregnancies with the given AFP level divided by the proportion of the unaffected pregnancies with the same level of AFP (9). The likelihood ratio is estimated from the ratio of the heights of the two log Gaussian frequency distribution at the given AFP level.
When the risks for Down syndrome based on maternal age and MSAFP level were combined, the detection rate of Down syndrome pregnancies approached 25-33%, at a false positive rate of 5%.(3, 9, 30).
Total hCG
In 1987 Bogart et al described an association between elevated second trimester hCG levels and pregnancies with fetal Down syndrome(6).
Human Chorionic Gonadotropin is a glycoprotein with a molecular weight of 47000 daltons composed of two subunits, alpha and beta. The alpha subunit is essentially identical to the alpha subunit of other pituitary peptide hormones. Specific biological activity is conferred by the beta subunit.
HCG is first produced by the trophoblast of the blastocyst and in later pregnancy by the chorion and placenta. Serum hCG levels increase exponentially between 3-10 weeks of pregnancy. Levels reach a peak during the first trimester (about 100000 mlU/ml) and decline during the second and third trimesters.
Studies have reported elevated second trimester hCG levels varying from 2.04 to 2.5 MoM or greater. A geometric mean MoM for Down's syndrome pregnancies determined from the results of 18 studies comprising a total of 559 Down's syndrome cases was 2.03 (7).
Wald et al retrospectively examined hCG levels in 77 Down syndrome pregnancies and, using maternal age and hCG levels, estimated a 60% detection rate at a false positive rate of 6.7%. Crossley et al reported similar findings. At a cut-off risk of 1/380 Kevin Spencer (5) et al found a 57.9% detection rate at a false positive rate of 8.5%.
Unconjugated estriol (Ue3)
Another marker, unconjugated estriol,(uE3) at first studied by Canick and co-workers, shows a correlation between low uE3 and trisomy 21, with a high level of correlation between AFP and uE3 (6). Estriol is produced by the placenta from maternal substrates (cholesterol and pregnenolone). Estriol diffuses from the placenta into the maternal blood where it can be measured as unconjugated uE3. In normal pregnancies uE3 levels increase from about 4nmol/L at 15 weeks gestation to about 40nmol/L at delivery.
Second trimester maternal serum uE3 levels in Down's syndrome pregnancies are approximately 75% of the values expected in normal pregnancies (8). A geometric mean maternal serum of 0.73 MoM derived from 363 cases of Down's syndrome in 11 studies has been reported (7).
Using maternal age and uE3 levels, at a cut-off risk of 1/380 Kevin Spencer et al (5) found a 45.7% detection rate at a false positive rate of 9.1%.
Free beta -hCG
In 1995, Eldar-Geva et al (19) showed that although the production of each subunit’s hCG messenger RNA is increased in Down syndrome pregnancies, beta subunit production is more markedly increased. This finding suggests that, the free beta-hCG subunit might be superior to intact hCG for Down syndrome detection. Free beta-hCG subunit concentrations average 0.5% of total hCG levels.
It has been claimed that the effectiveness of free beta-hCG methodologies is superior to that of total hCG in maternal serum screening (2,5,8,10,11,12).
In a multicentre study including 90 cases of Down’s syndrome pregnancies, Spencer confirms that in the affected pregnancies the free beta beta-hCG values were 2.06 times higher than the normal average (5). In this study, using maternal age and free beta-hCG levels, at a cut-off risk of 1/380, a detection rate of 61.0% at a false positive rate of 8.3%, was found.
Although, there is some concern about the stability of the free beta hCG since it has been demonstrated that the concentration of free beta-hCG increases significantly after only 6 hours at room temperature (13, 43). Also about the precise nature of the analyte, because it has been shown that different antibodies interact with free beta-hCG differently (14,15).
Another source states that, within precise limits of the technique:- Whole blood can be conserved at room temperature for 24 hours.
- Serum can be conserved at room temperature for 3 days.(16)
Other serum markers - Future perspectives
- Pregnancy-Associated Plasma Protein A (PAPP-A).
First-trimester free beta-hCG and PAPP-A screening for Down syndrome can achieve detection rates as high as those associated with AFP and hCG or AFP, hCG and uE3 in the second trimester. Prospective studies are needed to further assess first-trimester screening (17,18). - Inhibin-A.
This marker, is used in a four-marker test and a reduction of the false positive rate was detected (20,21).
- Urea - resistant neutrophil alkaline phosphatase and
- Ca - 125
are under study.
Under study are, as well, other questions such as:- Is maternal serum triple screening a better predictor of Down’s syndrome in female than in male fetuses ? (57) or
- Are Down’s syndrome fetuses detected through maternal serum screening similar with those remained undetected? (58)
Under investigation is a new triple screen test for Down syndrome combining urine analytes and serum AFP (59).
Also, there are enthusiastic reports (60) on screening for Down syndrome at 14 weeks of pregnancy .
Screening test combinations
Down’s syndrome screening relies on combining maternal serum markers results with maternal age in a complex mathematical algorithm using commercially available software programs. The test is interpreted as positive or negative according to whether or not the risk exceeds a fixed cut-off point. However, there is considerable debate about which analytes are most effective and which combinations provide the best detection rate.
The problem can be crystallized into two questions:- Does estriol significantly improve detection rates? and
- Is free beta hCG superior to total hCG ?
- total hCG, AFP, UE3; and free beta-hCG, AFP, uE3
- free beta-hCG and AFP.
As far as the contribution of MSAFP to second-trimester biochemical screening is concerned, although this contribution is marginal (a detection efficiency of almost 25%-33% when combined with maternal age), the role of the analyte appears secure as a component of Down’s syndrome screening because of its widespread use in screening for NTD at this stage of pregnancy (22)
Does estriol significantly improve detection rates?
There are several conclusions recommended against using uE3 levels as a marker for prenatal Down syndrome screening (20,23).The addition of uE3 to the screening protocol has not consistently improved detection rates, possibly because of its high correlation with AFP (6,24). In another study (28), the addition of uE3 lowered the detection rate of Down syndrome pregnancies with only a small and insignificant effect on the false-positive rate.
At the same time, several other sources rely on the usefulness of uE3 as a serum marker (25,26 27). In one study (29), if uE3 was omitted, the detection rate decreased from 60 to 48%. In another study (30), the exclusion of uE3 from the screening protocol would have reduced the detection rate for the same false-positive level.
In a recent study (12), at a cut-off risk of 1:380, the detection rate of Down’s syndrome was comparable between the double test (74%) and the triple test (65%, not significantly different). At least the double test is not worse than the triple test. Since the addition of uE3 to the double test adds no advantage in the detection rate, the double test is preferred to the triple test, being at a lower running cost.
Free beta-hCG versus total hCG
In a report (37) directly comparing free beta-hCG with hCG in the same sample set it was concluded that free beta-hCG analysis within a Down syndrome screening protocol provides a better detection efficiency than does hCG, especially in the earlier weeks of the second trimester. Further, in a multicentre study in which both free beta-hCG and total hCG were also run on the entire data set, free beta-hCG was shown to improve the detection efficiency by 10% over that of total hCG (39).
Another study (40), comparing the level of free beta-hCG in 480 Down syndrome cases with the level of hCG of a similar sized group, reported a median free beta-hCG MoM of 2.64, being significantly different from the median MoM of 2.07 of total hCG. In this study it was also demonstrated the detection efficiency in both retrospective and prospective data. The detection efficiency is 82.0% prior to 17 weeks of gestation and 70.2% at or after 17 weeks of gestation. The false-positive rate for the prospective study was 3.8%.
In a similar study (41) it was reported a 58% detection efficiency in patients under 35 years of age.
The improved detection efficiency of free beta-hCG over hCG suggests that median values in affected cases should be significantly higher than median values of intact hCG (40).
In agreement with this statement there are also data gathered by a recent study performed in Geneva (12).In this study, the median concentration measured was 1.94 MoM for total hCG and 2.68 MoM for free beta-hCG. The wider separation between the median concentrations in affected and unaffected pregnancies explains the higher detection rate of Down’s syndrome pregnancies reported with free beta-hCG as compared with total hCG. The protocol of that study using free beta-hCG, offered a 74% detection rate at a 6.6% number of positive cases versus a 65% detection rate at a 8.0% number of positive cases for total hCG . Therefore, for a fixed false-positive rate, an 8-10% higher detection rate can be predicted. The significant reduction of the number of false positive cases is an important advantage of the screening protocols using free beta-hCG. Lowering the false positive rate is a critical issue in patient care, as it means less parental anxiety, fewer unnecessary invasive tests and reduced costs.
The conclusion that the use of free beta-hCG in screening protocols is associated with a higher Down syndrome detection rate at a lower false-positive rate is also found in many other studies (5, 10, 31,32, 42).
As a conclusion, from the comparison of the triple and double tests for the detection of Down’s syndrome pregnancies, the combined use of free beta-hCG and AFP instead of total hCG, AFP and uE3, is a better screening test because it significantly decreases the number of false positive cases at a lower running cost. The addition of uE3 adds no further advantage to the double test.
Although, in a similar study comparing triple test with double test, Kellner et al (25, 26), reached opposite conclusions.
In fact, the only apparent difference between these opposite conclusions is the lower cut-off selected (1:270 to 1:380) (26).
Considering the limited number of affected cases included, it is impossible to reach definitive conclusions concerning the detection rate of fetal Down syndrome. Additional, comparative studies are needed to clarify these points further.
This a reason why in the literature we encounter studies using the double test (31, 32) and others using the triple test (33-36).
Factors affecting the accuracy of the screening
The importance of accurate gestation dating
Menstrual cycles may be irregular and it is even possible for a period to be missed so that the estimated date of conception may be out by as much as 4 weeks. Therefore it is necessary to check that gestation dates are correct before screening takes place, since the measured concentrations of the serum markers vary with gestational age (22,44). Choice of method for gestation will therefore have a large influence on the reliability of the calculation of the risk.
For the purposes of screening, ultrasound estimation is the best way to determine gestational age. Using biparietal diameter measurement (BPD) is preferable, because fetuses affected with Down syndrome often have shorter femurs and other long bones. Using femur length measurement instead of BPD could date the pregnancy earlier, thereby reducing the detection rate of Down syndrome.
Other maternal factors in Down’s syndrome screening
The influence of maternal weight.
Maternal weight can have a significant effect on the screening process (45, 46). AFP, being an overspill protein having no real function in maternal blood, has no regulated levels. Therefore, if the volume of distribution is greater, the concentration of AFP will be smaller (44). A similar effect but with a lesser degree of correlation is found for hCG (44, 47) but there is no correlation for uE3 (45).
Thus, a weight correction formula eliminates individual weight-related differences (48).
Smoking.
Adjusting the serum markers used for Down’s syndrome screening for the effect of maternal smoking has a small effect on overall screening performance (49).
Diurnal variation of serum markers.
Both a cross-sectional and longitudinal study were performed to investigate whether or not the collection time should be taken into consideration. If the levels of these markers routinely fluctuate during the day, they could effect a patient’s risk calculation for fetal Down syndrome. Using either study design, no significant effect was in the median MoM levels of the screening markers as a function of the time of day (50).
Effect of parity.
In one study (51, 52),cases were grouped by maternal parity. The mean MoM of MSAFP, uE3 and hCG was determined for each group. A correction factor was derived for each parity group and applied to the database. The results were: parity significantly affected the mean MoM of hCG but did not affect the values for uE3 or MSAFP. Application of a parity correction factor for hCG increased the Down syndrome detection rate in women who had two or more pregnancies from 71% to 82% without increasing the overall screening-positive rate.
Screening in multiple pregnancies.
In a study, conducted by Spencer et al. (53), on average, the levels of MSAFP and free beta-hCG were twice as high in twins and over three times as high in triplets
Possible genetic predisposition to abnormal levels.
Dar et al. concluded that there is a predisposition for abnormal levels of serum markers that is influenced by genetic or environmental factors. More data are needed before an accurate adjustment based on previous results can be made.
Differences between races or ethnic groups.
A study (55), evaluating data from more than 21000 pregnancies to determine the extent of race-specific differences in median concentrations, found that these medians were all significantly different. Differences remained significant even when data were corrected for patients weights. For each analyte, the extent of the variation was not the same at different gestational ages
Insulin-dependent diabetes mellitus.
In insulin-dependent diabetes mellitus patients, MSAFP levels have to be adjusted, since that has an effect on increasing the detection rate (56).
Practically informing patients of screening results
A health care professional should inform the patient of her test results, whether screen negative or screen positive.
Patients with screen positive results- The screening test gives the chance of carrying an affected fetus. It is not a diagnostic test which can give a definite answer about whether the fetus has the anomaly.
- A positive screening result does not mean that there is a problem, only that there is an increased risk and additional diagnostic tests are offered.
- The most common outcome of a positive screening result is a normal baby.
- A negative result means that the risk of having a Down syndrome fetus is low enough that a diagnostic test is not proposed without the woman’s initiative.
- A negative screening result does not guarantee a normal baby.
- These screening programs screen only for certain birth defects. It is not a test for all birth defects.
Suggested screening protocol for Down's syndrome
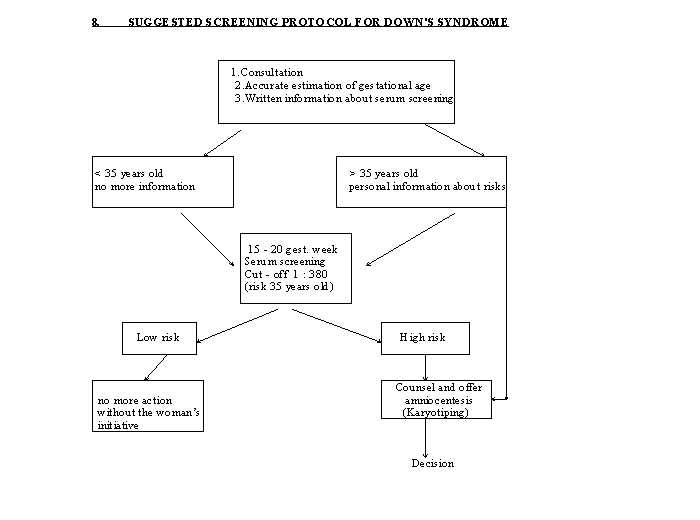
Suggested screening protocol for fetal Down syndrome (modified from Huch and Zimmermann).
Perspectives for DS screening in Albania
Down syndrome screening programs are not available in Albania so far. The value of developing in the future these screening programs is multilateral since they provide results for NTD, AWD and chromosomal anomalies.
The first and most important point against making these programs is the poor economic status of the country. The government is unable to give money. Besides, there is a lack of experience in this field.
At the very beginning an epidemiological study to determine the incidence of the anomaly in the country has to be performed. Then it is necessary to assess how much the Albanian society invests in these handicapped individuals and a detailed program of comparison can be offered to the government. If the program shall be seen as a priority, then a pilot study can be performed.
Albania has a strong centralized prenatal care system.
A pilot study in the capital Tirana and its surroundings could be performed, assessing the acceptability of the method by the population.
In Tirana, there is an equipped laboratory where the serum markers can be readily measured and also a Conserving Blood Center where blood and serum samples can be conserved.
A follow-up study can be performed due to the centralization.
What is really missing, is an initial fund for the amount of the kits to perform the pilot study.
We hope that with the available data from that study, proving the economical efficiency, the Albanian government will find the possibility of funding furthermore this practice throughout the whole country thus improving prenatal care.
Conclusion
The addition of maternal serum biochemical markers can improve screening sensitivity for fetal Down’s syndrome. Their best combination, in addition to maternal age, is found to be free beta-hCG + AFP, but still this remains controversial. The information must be very careful and individualized since for different patients risk factors are quite personal. Besides, people from different cultures react different to the philosophy of this screening.
References
1. Jackson LG et al A randomised comparison of transcervical and transabdominal CVS sampling N E J Med 1992; 594-8
2.Kazharine D.Wenstrom, MD et al. Free beta - hCG subunit versus intact hCG in Down syndrome pregnancies Obste Gynecol 1997; 90 : 370 - 4.
3. Cuckle et al Estimating a woman’s risk of having a pregnancy associated with Down’s syndrome using her age and serum AFP level Br J Obstet Gynaecol 1987 ; 94:387-402
4.Wald NJ et al. Antenatal screening for Down syndrome. J Med Screen 1997; 4(4): 181 - 7
5. Kevin Spencer et al Free beta -hCG in Down syndrome screening :A multicenter study of its role compared with other biochemical markers Ann Clin Biochem 1992; 29:506-518
6. Loncar J, Barnabei VM, Larsen JW Advent of maternal serum markers for Down’s syndrome screening Obstet Gynecol Survey 1995 APR; 50 (4) :316-320
7. Wald N et al Prenatal biochemical screening for Down’s syndrome and NTD.
Current opinion in obstet and gynecol 1992; 4:302-7
8. Olivier Dedeystere Interet du dosage de la sous-unite beta libre d’hCG dans le depistage prenatal de la trisomie 21 Immunoanal Bio Spec 1993 8, 177-179
9.George J Knight Glenn Palomaki James E Haddow. Use of MSAFP measurements to screen for Down syndrome. Clinical Obs Gyn, vol 31, No 2, June 1998.
10. Wenstrom KD, Owen J, Chu DC, Boots L Free beta-hCG subunit versus intact hCG in Down syndrome screening Obstet Gynecol 1997 Sep; 90 (3) :370-374
11. Milunsky A, Nebiolo LM, Bellet D. Maternal serum screening for chromosome defects:hCG versus its free beta-subunit. Fetal Diagn Ther 1993 Jul ;8(4) :221-224
12. P. Extermann, P. Bischof, P.Marguerat and B.Mermillod Second - trimester maternal serum screening for Down’s syndrome :free beta -hCG and AFP, with or without uE3, compared with total hCG, alpha-fetoprotein and uE3 Human Reproduction vol 13 n.1 pp.220-223, 1998
13. Stevenson H, Leslie H, Sheridan B. Serum free beta - hCG concetration increase in unseparated blood specimens Ann Clin Biochem 1993 ; 30 :99 - 100
14. Cole L, Kardana A Discordant results in hCG assays Clin Chem 1992 ; 38 :263 - 70
15. Cole L, Seifer D, et al Selecting hCG immunoassays : consideration of cross - reacting molecules in first trimester pregnancy serum and urine Am J Obstet Gynecol 1993 ; 168 : 1580 - 6
16. L. Malogrida, R. Rozenberg, Y. Giudicelli Depistage du risque de trisomie 21 au Chi de Poissy - 1. Au sujet de la stabilite de la beta hCG libre. Novotel, 12 juin 1996
17. Krantz DA et al. First - trimester Down syndrome screening: free beta-hCG and PAPP - A. Am J Obstet Gynecol 1996 Feb ; 174 (2) :612 - 616
18. Haddow JK, Palomaki GE, Knight GJ et al. Screening of maternal serum for fetal Down syndrome in the first trimester. N Engl J Med 1998 Apr 2 ; 338 (14) : 955 - 61
19. Eldar - Geva T, Hochberg A, deGroot et al. High maternal serum chorionic gonadotropin level in DS pregnancies is caused by elevation of both subunits messenger RNA level in trophoblasts. J Clin Endocrinol Metabol 1995; 80 : 3528 - 31.
20. Wald NJ, Densem JW, Knight PG Prenatal screening for Down’s syndrome using Inhibin - A as a serum marker Prenat Diag 1996 Feb; 16 (2) :143 - 153
21. Watt HC, Wald NJ, Huttly WJ. The pattern of maternal serum inhibin- -A concetrations in the second trimester of pregnancy. Prenat Diagn 1998 Aug; 18 (8) :846 - 8.
22.Lemay et al. Maternal serum screening for fetal Down syndrome, a retrospective study.Clin Chim Acta 238 1995 151 - 162.
23. J N Macri et al. Maternal serum Down syndrome screening :unconjugated estriol is not useful. Am J Obstet Gynecol 1990 : 162 :672 - 3
24. Reynolds T et al . The utility of unconjugated estriol in Down syndrome screening is unproven. Clin Chem 1993 ; 39 ; 2023 - 5
25. Kellner LH, Weiner Z et al. Triple marker (AFP, uE3, hCG) versus AFP + free beta - subunit in second - trimester maternal serum screening for fetal Down syndrome.: a prospective comparison study. Am J Obstet Gynecol 1995 Oct; 173 (4) :1306 - 1309
26. Kellner LH et al. The advantages of using triple marker screening for chromosomal abnormalities Am J Obstet Gynecol 1995 Mar ;172 (3) : 831 - 836
27. Knight GJ, Palomaki GE, Haddow et al. Hcg and the free beta - subunit as screening tests for Down syndrome. Prenat Diagn 1998 Mar; 18 (3) :235 - 45
28. David M, Merksamer R, Israel N, Dar H. Unconjugated estriol as maternal serum marker for the detection of Down syndrome pregnancies. Fetal Diagn Ther 1996 March; 11 (2) :99 - 105
29. MacDonald ML, Wagner RM, Slotnich RN. Sensitivity and specificity of screening for Down syndrome with AFP, Hcg, Ue3 and maternal age. Obstet Gynecol 1991 Jan ; 77(1) :63 - 68
30. Goodburn SF et al. Second trimester maternal serum screening using AFP, hCG and Ue3. Prenat Diagn 1994 MAY ; 14(5) .391 - 402.
31. Hsu JJ, et al. Down syndrome screening in an asian population using AFP and free beta - hCG: a report of the Taiwan Down syndrome screening group. Obstet Gynecol 1996 Jun; 87 (6) :943 - 947
32. P. Rozenberg - A. Gillet Depistage biologique et echographique de la trisomie 21. Realites en Gynecologie - Obstetrique N 8 - Janvier 1996 Cigarette smoking and levels of MSAFP, uE3 and hCG impact on Down syndrome screening. Obstet Gynecol 1993 May;81 (5 Pt 1) :675 - 678.
33. Salonen R et al. Maternal serum screening for Down’s syndrome on population basis. Acta Obstet Gynecol Scand 1997 Oct;76 (9) :817 - 821
34. Mancini G et al. Maternal serum markers. Estimation of the risk of Down syndrome :a prospective study. Int J Clin Lab Res 1994 ;24(1) :49 - 53.
35. McDuffie et al . Prenatal screening using maternal serum AFP, hCG and uE3:two year experience in a health maintenance organization. J Matern Fetal Med 1996 Mar; 5 (2) :70-73
36. Benn PA et al. Maternal screening for birth defects: results of a Connecticut regional program. Conn Med 1996 Jun; 60 (6) 323 - 327
37. Macri J N et al. Enhanced Down syndrome detection with free beta - hcg Am J Hum Genet 51(suppl), A418 1992
38. Muller F, Savey L, et al. Maternal serum hCG level at 15 weeks is a predictor for preeclampsia Am J Obstet Gynecol 1996 Jul ; 175 (1) :37 - 40
39. Spencer K, Makri J N. Early detection of Down syndrome using free beta - hcg Ann Clin Biochem 1992, 29 ; 349 - 350.
40. James N. Macri, K. Spencer et al. Maternal serum free beta - hCG screening: Results of studies including 480 cases of Down syndrome Prenatal Diagnosis, Vol 14; 97 - 103, 1994
41. Haddow J. E. Palomaki G. E. Knight G. J. et al Prenatal screening for Down syndrome with use of maternal serum markers. N. Engl J Medic 1992 327, 588 - 593
42. Kevin Spencer, Paul Carpenter. Prospective study of prenatal screening for Down’s syndrome with free beta - hCG BMJ 1993; 307:764 - 9
43. Andrew Kardana and Laurence A. Cole Polypeptide nicks cause erroneous results in assays of hCG free beta - subunit Clin Chem 38/1, 26 - 33 1992
44. Reynolds T. Practical problems in Down syndrome screening. What should we do about gestation dating ? What is the effect of laboratory imprecision ? Commun Lab Med 1992 ;2 ; 31 - 8.
45. Reynolds T et al The effect of weight correction on risk calculation for Down’s syndrome. Ann Clin Biochem 1991; 28:245-9
46. Watt HC, Wald NJ. Alternative methods of maternal weight adjustment in maternal serum screening for Down syndrome and NTD. Prenat Diagn 1998 Aug; 18 (8) :842 - 5
47. Wenstrom KD, Owen J, Boots L, Ethier M. The influence of maternal weight on hCG in the multiple - marker screening test for fetal Down syndrome. Am J Obstet Gynecol 1995 Oct; 173 (4) :1297 - 1300
48. Bartels et al. Adjustment formulae for MSAFP, hCG and uE3 to maternal weight and smoking. Prenat Diagn 1993 Feb; 13 (2) :123 - 130
49. Palomaki GE et al. Cigarette smoking and levels of MSAFP, uE3 and hCG impact on Down syndrome screening. Obstet Gynecol 1993 May;81 (5 Pt 1) :675 - 678.
50. Palomaki GE et al. Second - trimester diurnal variation of MSAFP, Hcg and uE3: is it present and does it affect the prediction of a patient's risk for fetal Down syndrome? Prenat Diagn 1994 Oct; 14 (10) : 947 - 951
51. Wenstrom KD, 0wen J, Boots L. Effect of parity correction on Down syndrome detection by the multiple - marker screening test. Am J Obstet Gynecol 1996 Oct ; 175 (4 Pt 1):1004 - 1007
52. Larsen SO et al. Inclusion of serum marker measurements from a previous pregnancy improves Down syndrome screening performance. Prenatal Diagn 1988 Jul; 18 (7) :706 - 12
53. Spencer K, Salonen R, Muller F. Down syndrome screening in multiple pregnancies using AFP and free beta - hcg. Prenat Diagn 1994 Jul ; 14(7) : 537 - 542.
54. Dar H, Merksamer R, et al. Maternal serum markers levels in consecutive pregnancies: a possible genetic predisposition to abnormal levels. Am J Med Genet 1996 Jan 11: 61 (2) :154 - 157
55. Benn PA, Clive JM, Collins R. Medians for second - trimester MSAFP, Hcg and Ue3; differences between races or ethnic groups. Clin Chem 1997 Feb; 43 (2) :333 - 337
56. Kramer RL et al. Effect of adjustment of MSAFP levels in insulin - dependent diabetes mellitus. Am J Med Genet 1998 Jan 13 ;75(2) :176 - 8
57. Ghdini A, Spang CY et al. Is maternal serum triple screening a better predictor of Down syndrome in female than in male fetuses ? Prenat Diagn 1998 Feb ; 18 (2) :123 - 126
58. Christiaens GC et al. Are Down syndrome fetuses detected through maternal serum screening similar to those remaining undetected ? Prenat Diagn 1996 May ;16 (5) :437 - 442.
59. Bahado - Singh RO et al. New triple screen test for Down syndrome: combined urine analytes and serum AFP. J Matern Fetal Med 1998 May - Jun; 7 (3) :111 - 4
60. Wald NJ, Watt HC, Haddow JE, Knight GJ. Screening for Down syndrome at 14 weeks of pregnancy. Prenat Diagn 1998 Mar, 18 (3) :291 - 3.
61. Sinosich MJ et al. Triple test for prenatal detection of trisomy 18. J Obstet Gynaecol Res 1996 Feb; 22 (1):57 - 60.
62. Barkai G, Goldman B, Ries L, Cuckle H. Effect of gravity on maternal serum markers for Down syndrome. Prenat Diagn 1996 Apr; 16 (4) ; 319 - 322.