11th Postgraduate Course for Training in Reproductive Medicine and Reproductive Biology
Injectable Contraceptives for Women
Adapted from: d'Arcangues C, Snow R. Injectable contraceptives.
In: Rabe T, Runnebaum B, eds. Fertility Control-Update and Trends. Springer-Verlag Berlin 1999: 121-149.
Introduction
An estimated 16 million women throughout the world are currently relying on injectable steroids for contraception. The choice of injectable methods includes products effective for 3 months, 2 months, or 1 month (Table 1). Although little known in Europe, these methods represent the third most prevalent form of reversible contraception worldwide. Trend estimates suggest that this number is rising, due to the reassuring World Health Organization (WHO) data regarding cancer risk, and the recent approval of the 3-monthly injectable depo medroxy-progesterone acetate (DMPA, or Depo-Provera) by the United States Federal Drug Administration (USFDA).
DMPA was developed in 1954 by the Upjohn Company for treatment of endometriosis and habitual or threatened abortions. In the early 1960s it was noted that women receiving DMPA for premature labor subsequently had a marked delay in return of fertility following delivery. This observation led to the development of DMPA as a fertility-regulating agent. In the mid-1960s, Upjohn requested a contraceptive product license for DMPA in many countries, and received approval to market it as a contraceptive in most of them. Administered as a dose of 150 mg DMPA in 1 ml aqueous suspension, the injection is given every 90 days deep into the gluteal or deltoid muscles, and one injection inhibits ovulation for at least 14 weeks. It is estimated that 13 million women are currently using DMPA, and the method is marketed in more than 90 countries worldwide.
As the 3-monthly injectable DMPA was promoted and brought to market by Upjohn, several alternative injectable contraceptive formulations were developed and underwent partial clinical evaluation. Few of these products gained widespread use other than norethisterone enanthate (NET-EN), which is given as an oily preparation intramuscularly at a dose of 200 mg every 60 days. Current users of NET-EN total approximately 1 million. The principal distinctions between DMPA and NET-EN are their durations of effectiveness and the incidence of drug-induced amenorrhea, and little else typically determines prescriptive practice. Nonetheless, given slight differences in the steroid derivation of the two drugs, experience of different side-effects with the two methods (e.g., weight gain or hirsutism), cannot be ruled out; however, most comparative data show few clinical differences other than duration of action.
The development of monthly, combined injectables actually preceded development of DMPA, with the first formulation tested in 1963 (Siegel 1963). Numerous products underwent partial development, clinical evaluation (and registration) in select countries. In the 1970s, motivated by interest in ensuring availability of an injectable that would not cause the disruption of menstrual bleeding commonly associated with DMPA and NET-EN, the Task Force on Long-acting Systemic Agents for Fertility Regulation, Special Programme of Research, Development and Research Training in Human Reproduction (HRP), WHO, undertook a review of all available combined, monthly preparations. They decided to optimize the dosage and the progestogen/ estrogen ratio of two combination formulations, Cyclofem and Mesigyna, and to oversee their clinical evaluation.
Cyclofem consists of 25 mg depot medroxyprogesterone acetate plus 5 mg estradiol cypionate, in a microcrystalline suspension in a 0.5-ml aqueous solution. Mesigyna consists of 50 mg norethisterone enanthate plus 5 mg estradiol valerate in a 1.0-ml oily solution of castor oil and benzyl benzoate, 60:40. Both preparations are given by deep, intramuscular injection into the gluteal or deltoid muscles. The first injection is given during the first 5 days after the onset of menses, or before the sixth week postpartum among women not breast-feeding. The second, and subsequent injections, are given 30±3 days from the last injection. It is estimated that approximately 1.5-2 million women currently use once-a-month injectables, mainly in Latin America and China.
Two additional monthly, combined injectable methods warrant mention. Deladroxate (commercially labelled as Perlutan, Topasel, Agurin, Horprotal and Uno-Ciclo in various countries) is a combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate, and is available in many Latin American countries and Spain. The method is highly effective, without a single pregnancy reported in large clinical trials (Koetsawang 1994). Although available since the 1960s, the method has not been studied as extensively as Cyclofem or Mesigyna. The original manufacturer withdrew support due to toxicological concerns with dihydroxyprogesterone acetophenide, and clinical evaluations continue to be published. A recent dose-finding trial compared the standard available dose of 150/10 with a lower dose of 90/6, and concluded the lower dose was equally effective (Coutinho et a1.1997).
Table 1. Formulation, Injection Schedule, and Availability of Injectable Contraceptives
| Formulation | Developer | Brand Name/Manufacturer | Injection Schedule | Availability |
| Progestin only: 150 mg depot medroxyprogesterone acetate (DMPA) | The Upjohn Company | Depo-Provera/Upjohn Megestron/Organon |
Every 3 months, 12 weeks, or 90 days |
Registered in over 100 countries; available in both public and private sectors. |
| Progestin only: 200 mg norethindrone (norethisterone) enanthate (NET EN) | Schering AG | Noristerata/Schering AG Doryxus/ Richter Gedeon Ltd. | Every 2 monthsb | Registered in over 60 countries; available in both public and private sectors. |
| Progestin + Estrogen: 25 mg DMPA + 5 mg estradiol cypionate |
Upjohn, WHO | Cyclofem, Cyclofemina, Novafem/Aplicaciones Farmaceuticas (Mexico), PT Tunggal (Indonesia), Lunelle/Upjohn (US) Cyclogeston/ PT Triyasa Nagamas Farma (Indonesia) |
Every month | Registered in 18 countries; available in both public and private sectors. |
| Progestin + Estrogen: 50 mg NET EN + 5 mg estradiol valerate |
WHO | Mesigyna, Norigynon/ Schering AG |
Every month | Registered in 35 countries. |
| Progestin + Estrogen: 150 mg Dihydroxy Progesterone Acetophenide + 10 mg estradiol enanthate |
Squibb Pharmaceutical Company |
Perlutan, Topasel, Agurin Horprotal, Uno-Ciclo/ Various manufacturers in Latin Americca |
Every month | Available in pharmacies in many Latin American countries and Spain; generally not available in public family planning programs. |
| Half-dose: 75 mg Dihydroxy Progesterone Acetophenide + 5 mg estradiol enanthate |
Anafertin, Yectames/ Various manufacturers in Latin America |
Every month | Latin America | |
| Progestin + Estrogen: 250 mg 17µ -hydroxy- progesterone Caproate + 5 mg estradiol valerate |
Chinese researchers; Squibb Pharmaceutical Company |
Chinese Injectable No. 1 | Every month, 2 injections in first month |
China |
a Called Norigest in Pakistan
b Alternative, less effective schedule: every 2 months for 6 months and then every 3 months
Sources: Population Reports (1995), WHO (unpub.)
Chinese Injectable No. 1, consisting of 250 mg 17-hydroxyprogesterone caproate and 5 mg estradiol valerate in an oily solution, is widely available in China. A comparative phase-III trial of Chinese Injection No. 1, Cyclofem, and Mesigyna was conducted in China in collaboration with the WHO in the late 1980s (Sang, Shao, Ge et al. 1995). The monthly injection schedule (30±3 days) of Chinese Injectable No. l was aborted when it was noted that this schedule was associated with an unacceptably high failure rate. Thereafter, the injection schedule was modified to a different schedule currently in use in China. Two initial injections are administered 10 days apart, and then subsequent injections are administered either 10-12 days following the next menstrual bleed, or 28 days following the last injection if no bleeding has occurred. The modified injection schedule is associated with a failure rate of 0.8 pregnancies per 100 woman-years (Sang, Shao, Ge et al. 1995). Informal reports suggest that use of this injection is declining in China, as alternative methods are promoted by the State Family Planning Commission. The method is also found in neighboring countries, such as Myanmar and Cambodia, but is perceived to have a high failure rate (Sadana, 1999), possibly due to difficulties maintaining the injection schedule required for optimal effectiveness.
Pharmacokinetic and Pharmacodynamic Features
Following a first injection of DMPA, MPA levels are at maximum concentration within the first three weeks (5-22 days) post-injection, at 15-26 nmol/l, decreasing to <1.0 nmol/l in the majority of women by 90-190 days post-injection (Garza-Flores 1994) (Fig. 1). NET-EN levels fall more rapidly than MPA, falling below detectable levels in most women within 46-110 days after injection.
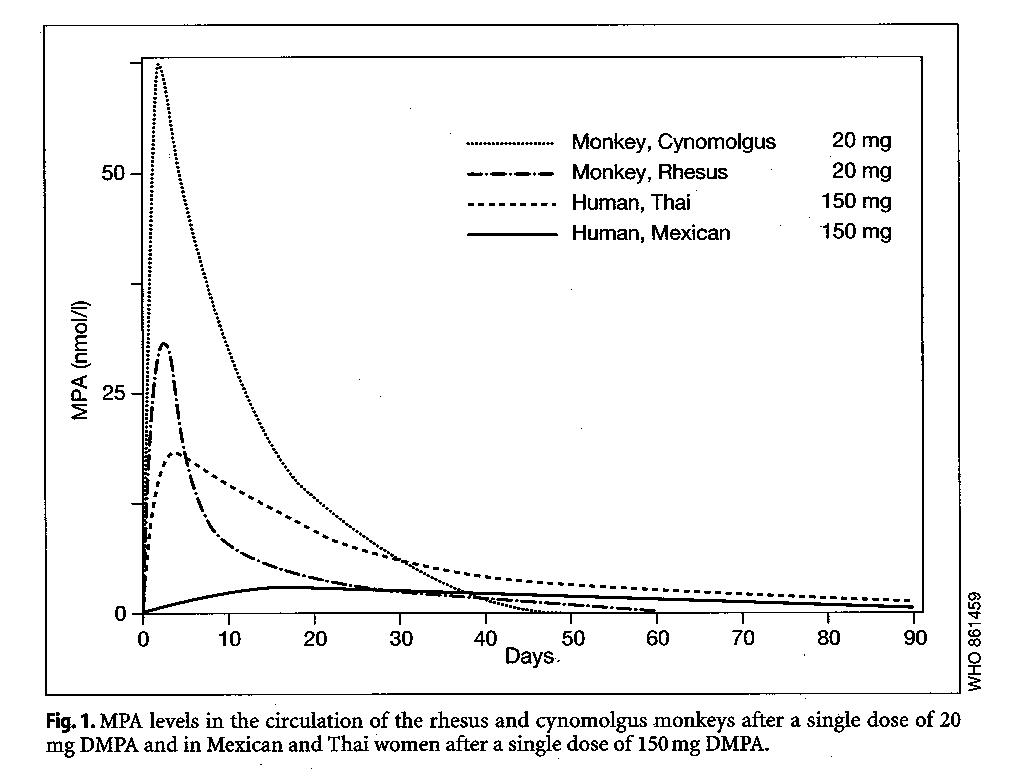 |
Ethnic variations in the pharmacokinetic pattern with DMPA have been reported from multi-center trials in different regions (Garza-Flores et al. 1994; Fotherby et al. 1980). A particularly rapid absorption and metabolism of DMPA has been noted among Thais, with high Cmax values followed by rapid elimination half-lives (GarzaFlores 1994). A comparatively slower absorption of DMPA has been observed among Mexicans, with blood levels an order of magnitude lower than some Thai women, and measurable concentrations of MPA observed 6 months after injection (Garza-Flores et a1.1994); limited available data from other ethnic groups suggest pharmacokinetic values between these two extremes.
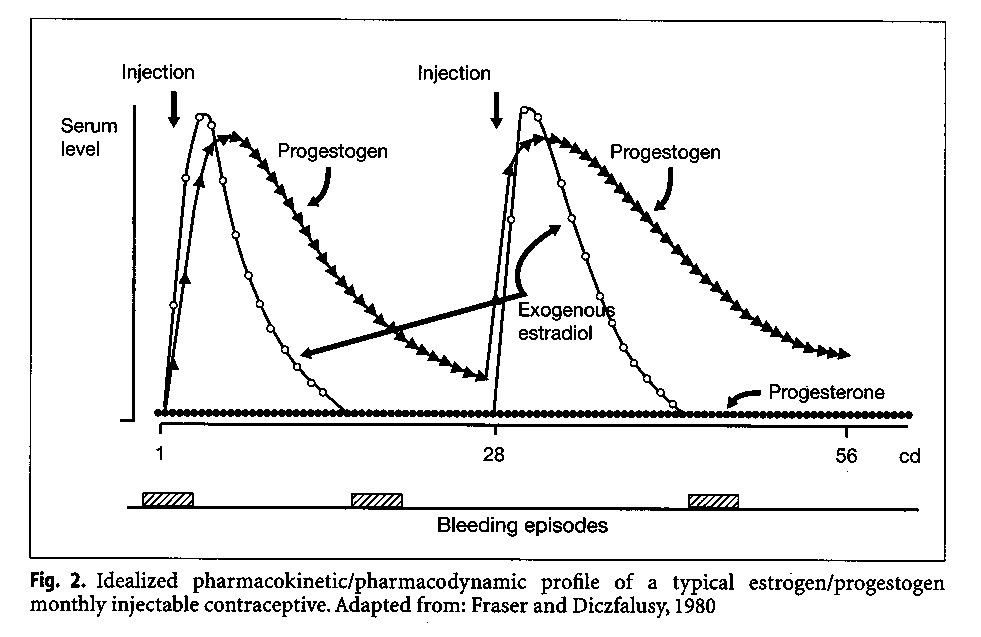
Pharmacokinetic and dynamic studies of the monthly injectables Cyclofem and Mesigyna were conducted in Stockholm (Aedo et a1.1985). Among users of Cyclofem the mean Cmax of medroxyprogesterone acetate was 2.9(2.4-3.7) nmol/l decreasing to 0.72(0.46-1.1) nmol/l 30 days after the last injection. Among users of Mesigyna, the mean maximum norethisterone levels were 10.1(6.4-15.8) nmol/l decreasing to 1.7(1.2-2.3) nmol/130 days after the last injection. From the schematic representation of the pharmacokinetic and pharmacodynamic profile of these preparations (Fig. 2), the biphasic nature of the steroid profile can be seen. Following the first injection a "combined phase" is characterized by a rise in progestogen and estrogen levels; when the estrogen level decreases approximately 2 weeks later, there is a "progestogen dominated" second phase.
 |
A prospective pharmacokinetic study of 17 Chinese women was designed to evaluate whether there was any measurable month-to-month accumulation of NET in the body during use of Mesigyna. NET concentrations in serum and saliva were measured after the 1st, 6th, and 12th monthly injection The mean peak concentrations of NET were 8.1 ng/ml, 6.6 ng/ml and 5.7 ng/ml, and the AUC's1-28 were 96.6 ng/ml-1/day, 84.6 ng/ml-1/day and 77.3 ng/ml-1/day, respectively. These data affirm that there is no accumulation of NET over 6 months or 12 months of treatment with Mesigyna (Sang 1994). Unpublished data on the pharmacokinetics of Cyclofem indicate a measurable month-to-month accumulation of MPA that continues for up to 6 months, and then levels off (Zhou et al. 1998). Both studies were conducted in Chinese women, and no similar data have been collected elsewhere. The latter observation is consistent with evidence from efficacy trials (discussed below), that the few pregnancies recorded with Cyclofem have occurred in the first several months of use.
Effectiveness
Current formulations of injectable contraceptives are highly effective. One injection of DMPA inhibits ovulation for 14 weeks, suppressing both FSH and LH. Cervical mucus is thickened, decreasing sperm penetration, the endometrium is atrophied with inactive glands, and there is decreased tubal motility. Pregnancies due to method failure are consistently low, with cumulative life-table rates of 0-0.1 percent at 12 months.
NET-EN is also highly effective. When given at 60-day intervals, the cumulative lifetable rates are 0.4% at 12 months, and 0.4% at 24 months.
Likewise, cumulative life-table pregnancy rates with Cyclofem and Mesigyna are between 0.0 and 0.2 at 12 months (WHO 1988b). In large, multi-center trials coordinated by the WHO, including more than 9000 women, all of the 13 pregnancies which occurred took place during the first few injection cycles, and no pregnancy occurred after the sixth injection (Koetsawang 1994).
Return of Ovulation
The return of ovulation following discontinuation of DMPA occurs approximately 5 months after the expected effect of the last injection, but the variance extends over several months. In a study of 14 women in Mexico with a duration of treatment lasting 1.2 to 5.0 years, the return of ovulation was 5.5±1.9 months (x±sd) after the expected effect of the last injection (Garza-Flores et al. 1985). The time to return of ovulation did not appear to vary with the duration of use, or with body mass index.
In the majority of women, ovulation does not occur as long as plasma levels of MPA are above 100 pg/ml (Ortiz et al. 1977). The return of ovulation occurs promptly thereafter, and individual variations in the return of ovulation appear to coincide largely with differences in metabolic clearance. In a pharmacodynamic study among Thai women, it was found that after a single dose of MPA (150 mg), eight subjects had varying times to return of ovulation (20-21 weeks in one subject, at sometime between 25 weeks and 33 weeks among four subjects, and not before 33 weeks in three subjects). However, the variance in the return of ovulation coincided with plasma concentrations of MPA. Seven of eight women had a return of ovulation only when MPA levels were no longer detectable; in one subject, ovulation occurred in the presence of very low, but detectable, levels of MPA (Lan et al.1984).
It should be noted, however, that a small comparative study of ovarian function following DMPA injection in India and Sweden reported that Indian women had a return of luteal function in the presence of higher doses (MPA>600 pg/ml) (Fotherby 1980).
When DMPA and NET-EN have been compared in the same population, with a common method for detecting ovulation, the 2-month injectable noresthisterone enanthate (NET-EN) has a comparatively shorter time for the return of ovulation, relative to DMPA (Garza-Flores et al. 1985). The comparative study among 24 Mexican women reported a more rapid re-establishment of menses following NET-EN than after DMPA, and an earlier return of ovulation (Fig. 3).
Data on the return of ovulation following use of Cyclofem and Mesigyna are limited, but available data indicate that it is more rapid than after use of DMPA or NET-EN. Three separate studies evaluated the return of ovulation after a Cyclofem treatment interval of three injections. In the second cycle after treatment, the authors reported that 55%, 88% and 71% of women, respectively, had ovulatory cycles (Fotherby et al. 1982; Aedo et al. 1985; WHO 1988b). In two similar studies following three injections of Mesigyna, 86% and 67% of women were ovulatory in the second cycle posttreatment (Aedo et al 1985; WHO 1988b). In a separate study among women who had used monthly injectables for 2 years, 60% of Cyclofem users, and 55% of Mesigyna users, ovulated at least once in the 90 days following treatment (see Bassol and Garza-Flores 1994).
In a small study (n=7) following the use of Delodrexate (in a 150/10 combination) for 1-2 years, ovulation returned within 60-90 days among all seven women. Among 16 women evaluated after at least 2 years of Delodrexate use, all returned to spontaneous ovulation 12-42 weeks after treatment (see Bassol and Garza-Flores 1994).
Return of Fertility
The return of fertility after DMPA use coincides with data on the return of ovulation: approximately 5 months. The largest prospective study followed 796 Thai users of DMPA who stopped using the method to have a planned pregnancy, and reported a median time to conception of 5.5 months after the expected effect of the last injection (Pardthaisong 1984). The same study included 427 women who stopped using oral contraceptives, and 125 women who had an IUD removed. The median time to conception after these methods was 3 months, and 4.5 months, respectively. An earlier life table analysis of 135 Thai women aged 18-37 years, who had discontinued DMPA, showed a cumulative pregnancy rate of 76.8% at 12 months (McDaniel and Pardthaisong 1973). When confined to 80 women, 18-25 years of age, the cumulative pregnancy rate was slightly higher (78.4%) at 12 months.
Among 69 Indian women discontinuing DMPA in order to plan a pregnancy, the cumulative conception rate at 12 months was 72.5% (ICMR 1986), a value only slightly lower than that observed for DMPA among Thai women (McDaniel and Pardtheisong 1973).
Studies of the return of fertility after NET-EN suggest values closer to DMPA. A prospective follow-up of 69 women who had used NET-EN for a minimum of six months reported a cumulative conception rate of 72.5 percent at 12 months (ICMR, 1986). However, the median time to conception was 7.8 months, longer than might be expected from data on the return of ovulation (i.e., 2.6 months) reported among Mexican women (Garza-Flores et a1.1985).
Individual and regional variations in the time to return of fertility after DMPA and NET-EN use are noteworthy; variances on the mean time until return of fertility are wide, and regional reports differ significantly. A retrospective review of clinical data on 363 Australian women suggested a mean interval from the end of the effective dose of DMPA to conception of 10.8 months (sd 9.7 months, median 9.2 months) (Fraser and Dennerstein, 1994), an interval twice as long as that reported from Thailand (Pardtheisong 1973). Regional differences may reflect underlying differences in the fecundability of different population groups (due to infection or cohort effects), differences in client motivation to conceive, or more fundamental differences in pharmacokinetics.
 |
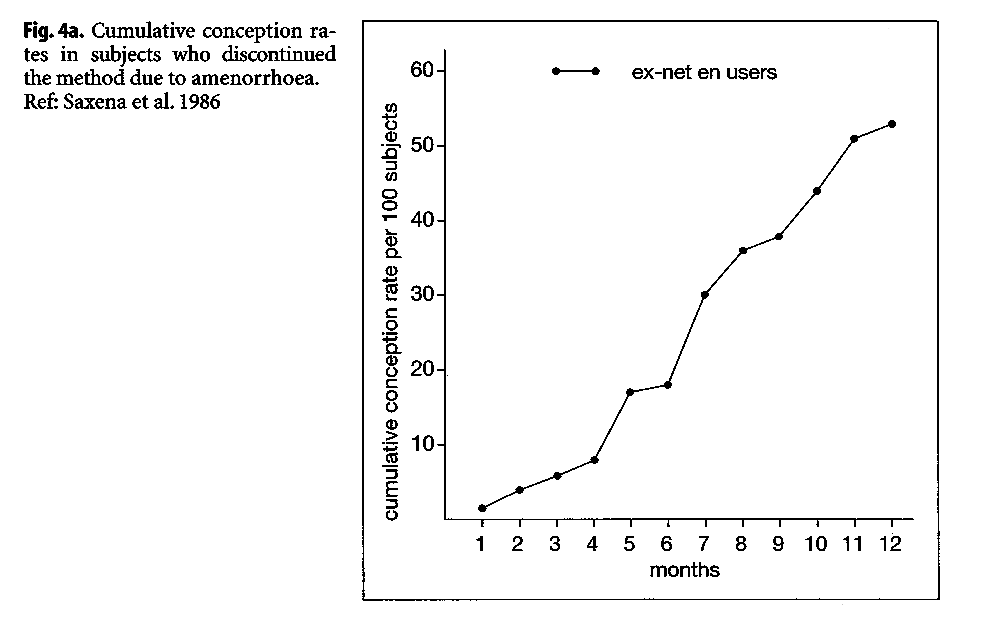
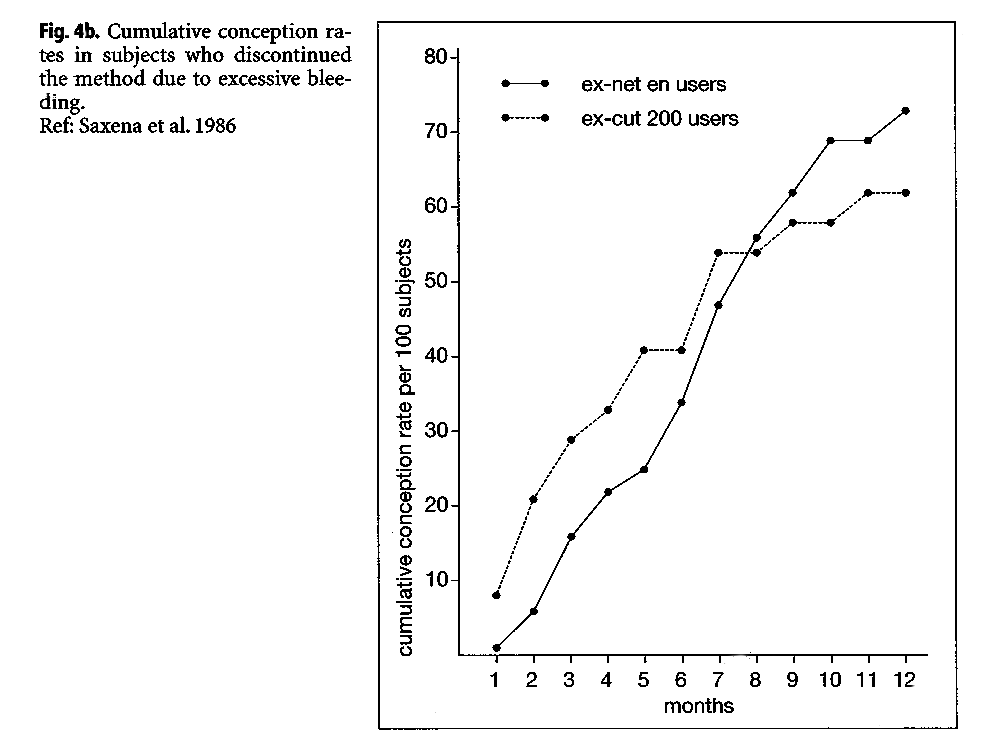
A study at the ICMR in India reported large differences in the cumulative pregnancy rates of ex-NET-EN users, when dividing them by bleeding experience while using the methods (ICMR 1986). Women discontinuing because of amenorrhea had a significantly slower cumulative conception rate over the ensuing 12 months (Fig. 4), relative to women who discontinued because of excessive bleeding. The 12-month conception rate for women discontinuing due to amenorrhea was 51%, compared with 73.5% among women who discontinued due to excessive bleeding.
 |
 |
The return of fertility following monthly injectables is considerably shorter than that observed for the progestogen-only injectables. Bahamondes et al (1997) reported observations on 70 Latin American women who had used between 1 and 19 injections of Cyclofem and discontinued to become pregnant. More than 50% were pregnant at 6 months, and the cumulative pregnancy rate was 82.9% at 12 months (Fig. 5). Return of fertility was not related to a woman's age, weight, or the number of Cyclofem injections. Among 19 women discontinuing use of Mesigyna to become pregnant, the cumulative pregnancy rate at 3 months was 100% (Sang, 1983). Keifer et al. reported that among 17 subjects who discontinued use of Delodrexate (150/10) to become pregnant, all conceived between 3 months and 36 months (see Bassol and GarzaFlores 1994).
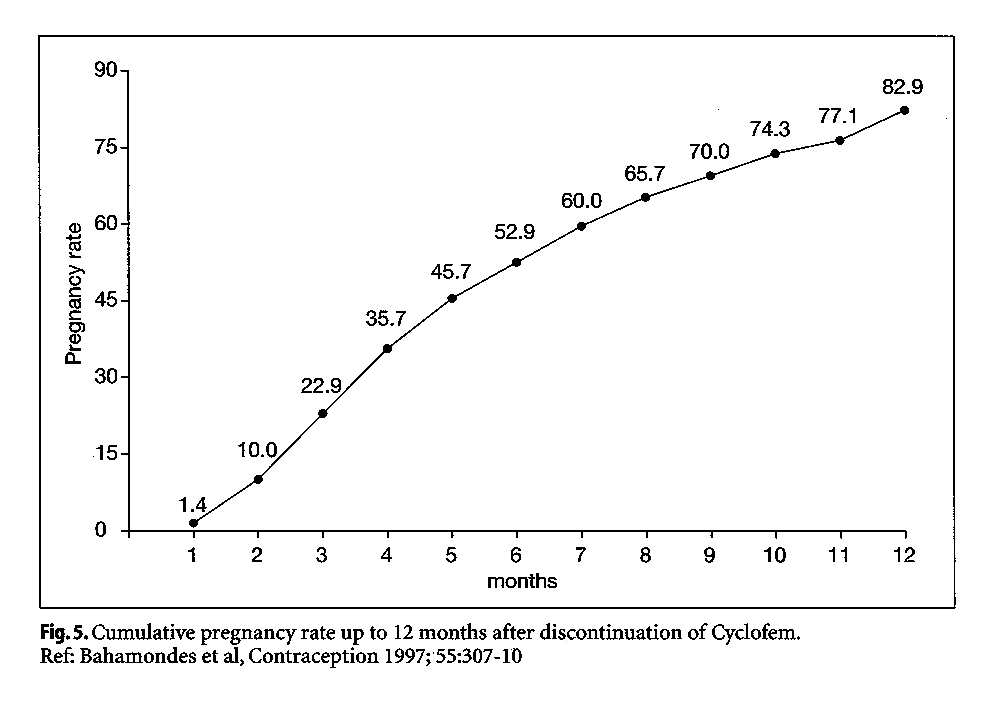 |
Women's Experience of the Methods
Irregularities in Vaginal Bleeding
The main side effect that women experience with injectable contraceptives is a disruption of their regular menstrual pattern. This is particularly the case with progestogen-only contraceptives, which induce an unpredictable pattern with episodes of prolonged and heavy bleeding, mostly during the first months of use. With DMPA, long periods of amenorrhea become increasingly frequent with continued use.
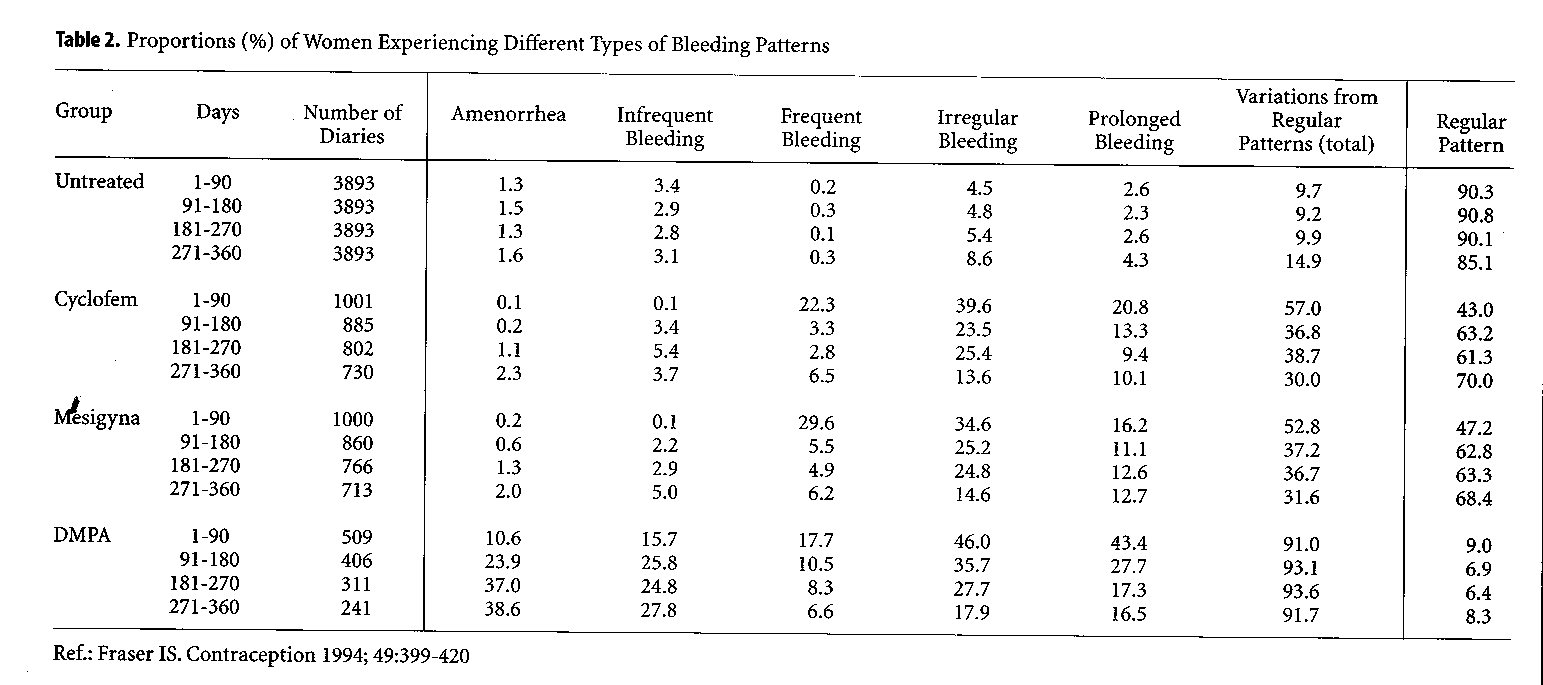
To illustrate these irregularities, the analysis of menstrual diaries from first year users of Cyclofem, Mesigyna and DMPA is shown in Table 2, in comparison with those of women using no method of contraception (Fraser 1994). During the first 3 months of DMPA use, close to half of the users experience irregular and prolonged bleeding. With continued use, these patterns become less frequent, while an increasing number of women experience infrequent bleeding or even complete amenorrhea. A similar trend is noted with the use of NET-EN, although the incidence of amenorrhea is not as high with this method.
As noted above, monthly combined injectables were developed to offer an alternative to women who could not tolerate such irregularities in their bleeding pattern. Following the first injection of these methods, both estrogen and progestogen levels rise. When the estrogen level decreases, i.e., approximately 2 weeks later (depending on the estrogen ester), a bleeding episode occurs which reflects this estrogen withdrawal. With monthly administration of the preparation, this phenomenon recurs on a monthly basis, giving the woman the experience of a first short cycle followed by regular monthly cycles. Data from multicenter studies (Table 2) (Fraser 1994) show that, with Cyclofem and Mesigyna, indeed two-thirds of the women will experience a regular monthly bleeding episode once the bleeding pattern is established. One-third will experience irregular or prolonged bleeding.
 |
It has been shown that even with progestin-only methods, the amount of blood loss is not a problem. In fact, the volume of blood loss is decreased compared with that measured during the natural untreated cycle, and anemia is not a significant risk associated with use of these methods. However, women do not tolerate the irregularity and unpredictability in vaginal bleeding which these methods induce.
Many women regard regular menses as the "pulse" of their reproductive health, indicative of their fertility and pregnancy status, and they associate an irregular menstrual pattern with ill-health. In addition, their menstrual pattern has sexual, socio-cultural, economic and sometimes religious implications, which make it an important factor affecting their everyday lives. It is, thus, not surprising that women do not easily tolerate disruptions of their menstrual patterns, and that menstrual disturbances are the main medical reason why women discontinue the use of injectable contraceptives (d'Arcangues et al. 1992).
The etiology of progestin-induced breakthrough bleeding is not clear. It differs from menstruation in that bleeding is focal and comes from small superficial veins and capillaries. Research has shown evidence of increased fragility of endometrial blood vessels, which probably results from disturbance of angiogenesis, and of reduced epxression of progesterone receptors at bleeding sites. In addition, the environment surrounding these blood vessles is altered. An increase in the number of endometrial leucocytes is usally observed, including T cells, macrophages and endometrial granulated lymphocytes. These cells can release free radicals, cytokines and proteolytic substances such as matrix metalloproteinases (MMPs). An imbalance between these MMPs and their inhibitors leads to rapid breakdown and collapse of the extra-cellular matrix, with resulting decreased endometrial perfusion and hypoxia. In addition tissue repair may be impaired, including the clotting mechanism that would normally restrict bleeding (Hickey and d’Arcangues 2001).
Various modalities are used by clinicians to alleviate the problem of menstrual disturbances, but few of those have been tested in placebo-controlled clinical trials (d’Arcangues 2000). Multi-country surveys of known practices for the management of menstrual disturbances in women using injectable contraceptives (Fraser 1983; Nutley and Dunson 1997; summarized in Table 3) testify to the lack of consensus, and of firm data available.
One placebo-controlled randomized clinical trial (WHO 1996b) was conducted to compare the effects of a 14-day treatment with either 50 µg ethinyl estradiol daily or 2.5 mg estrone sulfate daily, on DMPA-induced prolonged bleeding. Treatment with ethinyl estradiol was successful in stopping the bleeding episode in 93% of cases, compared with estrone sulfate and placebo, which had success rates of 76% and 74%, respectively. However, immediately after treatment, women given ethinyl estradiol had less bleeding but a more unpredictable pattern than the other two groups. Thus, treatment of DMPA-induced prolonged bleeding with ethinyl estradiol had a shortterm effect, but no beneficial effect on the acceptability of DMPA as a contraceptive method, and treatment with estrone sulfate was no different from placebo.
Amenorrhea itself is not well tolerated by some women as it gives them doubt about their fertility and their pregnancy status (Guzman-Garcia et al. 1997). A study (PiyaAnant 1998) was conducted among amenorrheic DMPA users to test the effect of switching to the monthly injectable Cyclofem. It showed that this approach will induce vaginal bleeding in 80% of women within 6 months. This approach has the benefits that a woman continues with a similar mode of contraception (i.e., injection), while the steroid load is actually reduced [3 x 25 mg vs 150 mg of DMPA for 3 months]; the traditional approach would have been to add supplemental oral estrogen to DMPA treatment (refer to Table 3).
Phase-I studies clearly demonstrated that the efficacy of once-a-month injectables was dependent on the estrogen/progestogen ratio (Garza-Flores et al. 1991). In particular, ovulation was no longer inhibited if the dose of estrogen was increased. Thus, it is not recommended to treat vaginal bleeding disturbances experienced by users of combined injectables with steroid hormones. Non-steroidal agents may be helpful, although their effectiveness has not been tested in controlled clinical trials.
Changes in Body Weight
A majority of women using DMPA gain body weight while using the method. Product information provided by the Upjohn Company (manufacturer of DMPA) describes average weight gain of 2.5 kg after 1 year of use (Product Information 1994). An early collaborative study of DMPA use among 3857 women in the USA reported a mean weight gain of approximately 2.3 kg after 1 year, 3.7 kg after 2 years, and 6.3 kg after 4 years (Schwallie and Assenzo 1973). Another early trial among 138 women in Calcutta reported mean weight gains above 4.0 kg at 1 year, and above 6.0 kg at 2 years (Mukherjee et a1.1980). A more recent WHO multicentre phase-III clinical trial of DMPA reported a mean weight gain of 1.5 kg per year (WHO 1986).
A study of NET-EN users after 1 year of use reported an average weight gain of 5.0 kg among 20% of clients, and an average weight loss of 5.0 kg among 15% (Howard et al. 1985).
Slight increases in body weight have been reported with use of both Mesigyna and Cyclofem in phase-III trials (Koetsawang 1994). In a 3-year clinical trial of Mesigyna, one-third of clients gained 3-8 kgs (Kesseru et al. 1994). In a multi-country study of Cyclofem from Indonesia, Jamaica, Thailand, and Tunisia, cumulative 12-month discontinuation rates for weight gain were only 4% in Thailand, and from less than 1.0% to 2.5% in the other three research sites (Hall et WHO 1994). In a study involving 3183 Cyclofem users in Brazil, Chile, Colombia and Peru, weight gain was found to be inversely proportional to weight at admission. Women weighing less than 50 kg at admission had a 7.7% weight increase at 13 months, compared to 1.7% for women weighing more than 64 kg at admission (Bahamondes et al. 1998).
Women's Perspectives
The principal patient complaint with DMPA and NET-EN is disruption of menstrual bleeding. DMPA has the highest discontinuation rate among modern, highly-effective contraceptives, and dissatisfaction with changes in menstrual bleeding is the most common method-related reason for discontinuation (d'Arcangues et al. 1992); this observation holds across different countries and ethnic groups.
Pretreatment counseling regarding expected side-effects with DMPA increases continuation rates (possibly by increasing tolerance for abnormal bleeding). A recent pre-DMPA counseling intervention trial in China reported 12-month discontinuation rates of only 11%, compared with 42% without counseling (Lei et al.1996).
While Cyclofem and Mesigyna provide better menstrual cycle control than DMPA, abnormal bleeding is still the main drug-related complaint with these methods, and the principal reason for discontinuation.
Headaches, mood changes and decreased libido have each been documented in association with DMPA use, but with wide variations in the frequency of reporting across different studies. A retrospective study of 363 women using DMPA in Australia reported sexual difficulties (loss of interest, dry vagina, dyspareunia), to be the third most important method-related side effect leading to discontinuation (after bleeding and headaches), and the principal complaint cited by women (43%) continuing with the method (Fraser and Dennerstein 1994).
Table 3. A survey of different approaches to management of menstrual disturbances in women using progestin-only injectable contraceptives (Adapted from Fraser, 1983 and Nutley & Dunson 1997)
| Regimens used for the management of prolonged or frequent light bleeding during use of injectable contraceptives | Regimens used for the management of prolonged amenorrhea occuring with injectable contraceptives |
| 1. Counselling, only: widely used in most centres | 1. Counselling only: almost all centres (ideally with abdominal and vaginal examination although this may not be easy in some cultures) |
| 2. Medical, including vaginal examination: If indicated; most centres. | 2. Urinary or serum pregnancy test: in selected cases |
3. Progestin
3.1 Early repeat contraceptive injection: several centres give next injection earlier than 90 days when troublesome bleeding occurs in DMPA users |
3. One or two short courses of "oestrogen": if greatly prolonged amenorrhea and patient unduly concerned
3.1 Combined oral contraceptive pill for one "cycle", |
4. One or more short courses of "oestrogen": sometimes advised
4.1 Various combined oral contraceptive pills given in different regimes: for example:4.1.1 One tablet daily for 14-21 days4.2 Ethinyl oestradiol 50 µg daily for 7-21 days |
4. Cyclical supplement oral oestrogen:
Any one of above regimens (3.1 to 3.4) daily for 5-21 days out of each month; previously used in several centres but no longer used as a routine in any centres. Still used very occasionally in individual cases in a few centres. |
| 5. Non-steroidal anti-inflammatory agents 20-800 mg three times a day for 5-7 days |
|
| 6. Supplements and placebos: 6.1 Iron supplements, usually oral 6.2 Vitamins C and K, multivitamin preparations 6.3 Calcium tablets 6.4 Diazepam 2 to 5 mg daily 6.5 Bed rest |
|
| 7. Diagnostic curretage: for further investigation of persistent bleeding. Very rarely needed and most clinicians had never needed to use it. More often mentioned when women were over age 35 to 40 or were very long-term users of injectable contraceptives. |
In a large clinical trial in Egypt, 10 percent of continuers, and 24 percent of women discontinuing Cyclofem and Mesigyna (and 10 percent of those continuing) reported daily headaches (Hassan et al.1994). In the same study 91 percent of continuers, and 91 percent of discontinuers reported no changes in libido with Cyclofem or Mesigyna.
Qualitative data on women's attitudes to injectable contraceptives underscore users' appreciation of the high method effectiveness, the possibility of using these methods secretly (the lack of paraphernalia), and the chance to use these methods for discrete intervals of protection (Guzman-Garcia 1997; Snow et al. 1997; Bledsoe et al. 1994). Women's discontent over the disruption of menstrual bleeding associated with these methods is a significant limitation; additional perceived negative attributes include the failure to have a rapid and predictable return of fertility after discontinuation, the lack of protection provided against STDs, the potential for provider abuse, and the lack of user-controlled reversibility within the injection interval (GuzmanGarcia et al. 1997; Snow et al. 1997).
Clinical Eligibility and Safety
Medical Eligibility Criteria
Current knowledge on injectable contraceptives justifies the use of these methods by many women. However, use is contraindicated for women with specific illnesses or conditions, and there are important differences in the suitability of progestogen-only preparations and combined methods. In 1994, 1995 and 2000, WHO convened meetings of experts to review the medical eligibility criteria of the most widely used contraceptive methods. Their recommendations relevant to injectable contraception are summarized in Table 4 (WHO 2000).
The basis for the WHO recommendations was: (a) indirect evidence or theoretical concern based on studies on suitable animal models, laboratory studies on humans, or analogous situations; (b) evidence derived from effects of the contraceptive method on women without the condition; and (c) evidence based on direct studies or observations of the contraceptive method on women with the condition. For combined injectables, there is little epidemiological data on their long-term effects as they have been introduced only recently. Thus, until such data become available, their suitability in the presence of severe pathologies was assessed conservatively, by analogy with combined oral contraceptive preparations.
Conditions affecting eligibility for the use of each contraceptive method were classified under one of the following four categories:
1. A condition for which there is no restriction for the use of the contraceptive method
2. A condition where the advantages of using the method generally outweigh the theoretical or proven risks
3. A condition where the theoretical or proven risks usually outweigh the advantages of using the method
4. A condition which represents an unacceptable health risk if the contraceptive method is used
These eligibility criteria were reviewed both to consider the initiation of use of a contraceptive method and also its continuation in case a medical condition develops during use of the method. When the criteria for these two situations differ and lead to different classification, these are indicated under "I" for initiation and "C" for continuation.
Cancer
DMPA exerts a protective effect against the risk of endometrial cancer. A collaborative hospital-based case-control study in 11 countries, coordinated by the WHO, reported an overall relative risk of 0.2 (95% confidence interval 0.1-0.8) (WHO 1991b). This protective effect appears similar, or even greater, than that associated with combined oral contraceptives, and the protective effect continues for at least 8 years after discontinuation of DMPA.
There is no epidemiological evidence of an association between DMPA use and the risk of ovarian cancer (Lumbiganon 1994), or between DMPA use and the risk of cervical cancer (La Vecchia 1994).
Several recent publications have contributed substantially to our understanding of the breast cancer risk associated with use of DMPA. A study coordinated by the WHO in Kenya, Thailand, and Mexico (WHO 1991a), and a second conducted in New Zealand (Paul et a1.1989) have provided convincing evidence that there is no overall risk of breast cancer with DMPA. However, both studies reported a small elevated relative risk in certain sub-groups. Current or recent use of DMPA, and women less than age 35 years at the time of cancer diagnosis, each showed a slight increased risk of breast cancer.
Table 4. WHO Eligibility Classification for use of progestin only (DMPA and NET-EN) and combined injectable contraceptives by key health condition. Ref: WHO 2000
| DMPA & NET-EN | Combined injectables | |
| Age | ||
| menarche to < 18 years | 2 | 1 |
| 18-45 years | 1 | 1 |
| ³ 45 years | 2 | 2 |
| Obesity | ||
| ³ 30 kg/m2 body mass index | 2 | 1 |
| Smoking | ||
| age < 35 years | 1 | 2 |
| age ³ 35 years, light1(< 15 cigarettes/day) | 1 | 2 |
| age ³ 35 years, heavy (> 15 cigarettes/day) | 1 | 3 |
| Parity | ||
| any | 1 | 1 |
| Pregnancy | N/A | N/A |
| History of high blood pressure during pregnancy | 1 | 2 |
| Past ectopic pregnancy | 1 | 1 |
| Breast feeding | ||
| < 6 weeks post-partum | 3 | 4 |
| 6 weeks to 6 month post-partum | 1 | 3 |
| ³ 6 months post-partum | 1 | 2 |
| Post partum | ||
| < 21 days | 1 | 3 |
| (in non-breast-feeding women) ³ 21 days | 1 | 1 |
| Post abortion (first trimester, second trimester, post-septic abortion) | 1 | 1 |
| Vaginal bleeding patterns | ||
| irregular pattern without heavy bleeding | 2 | 1 |
| with heavy or prolonged bleeding (includes regular and irregular patterns) | 2 | 1 |
| Unexplained vaginal bleeding (suspicious for serious underlying condition) - before evaluation |
3 | 2 |
| Severe dysmenorrhea | 1 | 1 |
| Breast disease | ||
| undiagnosed mass | 2 | 2 |
| benign breast disease | 1 | 1 |
| family history of breast cancer | 1 | 1 |
| cancer - current history of breast cancer | 4 | 4 |
| cancer - past and no evidence of current disease for 5 years | 3 | 3 |
| Cervical intraepithelial neoplasia (CIN) | 2 | 2 |
| Cervical cancer (awaiting treatment) | 2 | 2 |
| Cervical ectropion | 1 | 1 |
| Benign ovarian tumours (including cysts) | 1 | 1 |
| Endometrial, ovarian cancer | 1 | 1 |
| Uterine fibroids | 1 | 1 |
| Endometriosis | 1 | 1 |
| Trophoblast disease (benign or malignant) | 1 | 1 |
| Prior pelvic surgery | 1 | 1 |
| Pelvic inflammatory disease | ||
| (current or past, with or without subsequent pregnancy) | 1 | 1 |
| STIs (at increased risk, current or recent disease) | 1 | 1 |
| HIV/AIDS (high risk of HIV, HIV positive, AIDS) | 1 | 1 |
| Hypertension | ||
| history of - (where blood presure cannot be evaluated) | 2 | 3 |
| adequately controlled hypertension | 2 | 3 |
| systolic 140-159 or diastolic 90-99 | 2 | 3 |
| systolic ³ 160 or diastolic ³ 100 | 3 | 4 |
| vascular disease | 3 | 4 |
Table 4. Continued
| DMPA & NET-EN | Combined injectables | |||
| Multiple risk factors for arterial cardiovascular disease (such as older age smoking, diabetes and hypertension) | 3 | 3/4 | ||
| Deep Venous Thrombosis (DVT) | ||||
| history of DVT/PE | 2 | 4 | ||
| Pulmonary Embolism (PE) | ||||
| family history of DVT/PE | 1 | 4 | ||
| current DVT/PE | 3 | 4 | ||
| major surgery with prolonged immobilization | 2 | 4 | ||
| major surgery without prolonged immobilization | 1 | 2 | ||
| minor surgery without immobilization | 1 | 1 | ||
| Superficial venous thrombosis | ||||
| varicose veins | 1 | 1 | ||
| superficial thrombophlebitis | 1 | 2 | ||
| Ischemic heart disease (current or history of -) | 3 | 4 | ||
| Stroke (history of cerebro-vascular accident) | 3 | 4 | ||
| Known hyperlipidemias | 2 | 2/3 | ||
| Valvular heart disease | ||||
| uncomplicated | 1 | 2 | ||
| complicated (pulmonary hypertension, risk of atrial fibrillation, history of sub-acute bacterial endocarditis) | 1 | 4 | ||
| Diabetes | ||||
| hx of gestational disease | 1 | 1 | ||
| non-vascular disease | 2 | 2 | ||
| nephropathy/retinopathy/neuropathy | 3 | 3/4 | ||
| other vascular disease or diabetes of > 20 years' duration | 3 | 3/4 | ||
| Gall bladder disease (current or treated, asymptomatic) | 2 | 2 | ||
| History of cholestasis | ||||
| pregnancy-related | 1 | 2 | ||
| past combined oral contraceptive-related | 2 | 2 | ||
| Viral hepatitis | ||||
| active | 3 | 3/4 | ||
| carrier | 1 | 1 | ||
| Cirrhosis | ||||
| mild (compensated) | 2 | 2 | ||
| severe (decompensated) | 3 | 3 | ||
| Liver tumours | ||||
| benign (adenoma) | 3 | 3 | ||
| malignant (hepatoma) | 3 | 3/4 | ||
| Thyroid disease | ||||
| (simple goitre, hypothyroidism, hyperthyroidism) | 1 | 1 | ||
| Sickle cell disease | 1 | 2 | ||
| Iron deficiency anemia | 1 | 1 | ||
| Thalassemia | 1 | 1 | ||
| Epilepsy | 1 | 1 | ||
| Schistosomiasis (any stage) | 1 | 1 | ||
| Malaria | 1 | 1 | ||
| Tuberculosis | 1 | 1 | ||
| Drug interactions - commonly used drugs with affect liver enzymes | ||||
| antibiotics (griseofulvin, rifampicin) | 2 | 3 | ||
| anticonvulsants (phenytoin, carbamazeptine, barbiturates, primidone) | 2 | 3 | ||
| other antibiotics (excluding griseofulvin and rifampicin) | 1 | 1 | ||
| Headaches | I | C | I | C |
| non-migrainous (mild or severe) | 1 | 1 | 1 | 2 |
| migraine without focal neurologic symptoms | 2 | 2 | 2/3 | 3/4 |
| migraine with focal neurologic symptoms | 2 | 3p">3 | 4 | 4 |
As both of the above studies shared similar designs and methodologies, yet each alone lacked the statistical power to adequately substantiate and interpret the observed risk for these sub-groups, data from the two studies were combined for a pooled analysis (Skegg et al. 1995). The conclusion of the pooled analysis was that current or recent use of DMPA was the key factor associated with the increased risk observed in both sub-groups, and women who had started using DMPA within the previous 5 years were estimated to have a relative risk of 2.0 (95% confidence interval, 1.5-2.8). It was concluded that the increased risk previously observed for women diagnosed under the age of 35 years was probably due to the fact that younger women were more likely to have used DMPA recently.
Limited epidemiological data are available regarding the cancer risk associated with monthly, combined injectables. In a small retrospective study reviewing contraceptive histories of 267 women with breast cancer, and 1520 controls, the relative risk of breast cancer among users of monthly injectables was 0.83 (95% CI 0.46-1.5) (Asham 1990).
While early reports from Chile suggested an increased risk of invasive cervical cancer with monthly injectable use, further data from both Chile and Mexico did not support such an association. A further study in Latin America noted that users of injectable contraceptives (55% monthly, 43% 3-monthly) tended to have fewer sexual partners and initiated sexual life at a later age than non-users. Adjusting for these factors, the overall relative risk of cervical cancer among women who had used injectables was 0.8 (95% CI 0.5-1.2). However, women who had used injectables for at least 5 years had a relative risk of 2.4 (95% CI 1.0-5.7) (Herrero et al. 1990). These data are not conclusive, but underscore the need for further study.
Chronic parenteral toxicity studies of Cyclofem for 24 months in monkeys reported Cyclofem was nontoxic, hormonally active, and noncarcinogenic at all doses including 50 times the human dose (Cooksen 1994).
Metabolic Effects
DMPA and NET-EN have minimal effects on most metabolic parameters. Lipid changes are minor and without clinical significance. Following a first injection of DMPA, there is a decline in fasting triglyceride and cholesterol levels, with a rapid return to pretreatment levels at the end of the injection interval (Amatayakul et al. 1980). Most studies of DMPA and NET-EN find slightly higher levels of LDL-cholesterol and lower levels of HDL-cholesterol. In a multi-center study, 50 women who had used DMPA for 3-9 years were compared with 120 users of IUDs. Findings differed among centers, but consistently noted unfavorable changes of moderate magnitude (WHO 1993). No significant changes in liver metabolism have been observed (Fotherby 1980; Amatayakul et al. 1980), nor are there any measurable effects on blood pressure or coagulation factors.
Likewise, most studies of monthly, combined injectables find minimal metabolic effects. Multi-center studies of lipid effects (UNDP/UNFPA/WHO/World Bank 1997) indicate slight changes without clinical significance, and the observed changes revert to baseline promptly after method discontinuation. The observed changes in lipids were biphasic: during the combined phase, HDL cholesterol and apolipoprotein A were increased, while LDL cholesterol and apolipoprotein B were decreased. In the second "progestogen-dominated" phase of the injection interval, these trends reversed.
No significant effects on blood pressure, blood coagulation or liver function have been found with use of monthly, combined injectables. In a multi-country study of liver function among users of Mesigyna and Cyclofem, the WHO found that both injectables caused a small increase in bilirubin, but values remained within the normal range. A small reduction in alkaline phosphatase was also within the normal clinical range, and there were no measurable changes in liver enzymes (UNDP/ UNFPA/WHO/World Bank 1998).
Changes in Glucose Dynamics
DMPA use is associated with a significant, albeit small, shift in the insulin response curve to an oral glucose challenge (Spellacy 1974; Vermeulen and Thiery 1974). There is a significant rise in both glucose and insulin values in response to glucose challenge, and an increase in the insulin to glucose ratio, suggesting mild insulin resistance. Clinicians agree that these changes do not indicate a risk of inducing diabetes in normal women, but the method is contra-indicated for women with diabetes.
Data on the association between Cyclofem or Mesigyna and carbohydrate metabolism indicate only minor changes in glucose tolerance, with minimal changes in insulin response to an oral glucose challenge. These changes were numerically larger with Mesigyna, but were judged to be of no clinical significance for either injectable (UNDP/UNFPA/WHO/World Bank 1998).
Exposure in Utero
Despite the high efficacy of progestin-only injectables, exposure of infants to these drugs in utero occurs, when the first injection is inadvertently given in very early pregnancy. A large number of studies have investigated the effect of exposure to DMPA, while few data are available on NET-EN. Exposure to DMPA was not found to increase teratogenicity (Katz 1985). Follow-up of children over the 17 years following exposure failed to show an effect on long-term health and development (Jaffe 1990), intellectual development (Jaffe 1988), or on the sex-dimorphic behaviors of aggressiveness, assertiveness and physical activity (Jaffe 1989).
As Cyclofem and Mesigyna are both relatively new, as well as very effective methods, there are very few reports available on infants exposed to these drugs in utero. In the few cases that are documented (WHO 1988a; Hassan 1999), all pregnancies ended with the birth of full-term healthy infants after exposure to one or three injections of a combined injectable. Follow-up beyond the time of birth is not available.
Exposure of Breast-Fed Infants
Transmission of steroidal hormones through breast milk is variable. MPA levels in breast milk are close to maternal plasma levels and are still detectable 12 weeks after DMPA injection. In contrast, NET levels in breast milk are about one-third of maternal plasma levels and are rarely detectable beyond 8 weeks (Koetsawang 1982). DMPA use during lactation does not appear to affect milk volume and has only minimal effects on milk composition (WHO 1988a). It is estimated that breast-fed infants receive 0.25 µg/kg/day 3-4 weeks after injection, and that this level falls progressively to 0.08 µg/kg/day when the next injection is due (Johansson and Odlind 1987).
Exposure to DMPA through breast milk was not found to affect the adrenal and gonadal functions of infants (Virutamasen 1996), nor their growth and development (WHO 1994a; 1994b). Follow-up of exposed children into early adulthood also failed to show any adverse effect on long-term growth and development (Pardthaisong 1992).
Combined hormonal contraceptive methods are not recommended during breastfeeding in view of the well-documented deleterious effect of combined pills on milk output and composition, duration of lactation and infant growth (Croxatto 1983; Diaz 1983; WHO 1984). Consequently, no report is available on the effect of combined injectables on lactation and on the growth of breast-fed infants.
Emerging Questions: Bone Density, HIV Transmission
DMPA Effects on Bone Mineral Density
In 1991, a study from New Zealand reported that women who had used DMPA for at least 5 years showed significantly lower bone densities in both the femoral neck and the lumbar spine than premenopausal controls (Cundy et al.1991). The study was criticized for failing to control for smoking or prior oral-pill use, but it raised concerns about the impact of long-term DMPA use on bone density, and hence, fracture rates.
DMPA suppresses follicular estrogen production, and estrogen maintains bone density by slowing bone resorption. Studies of circulating estrogen among DMPA users suggest that, on average, estrogen levels are above those found among postmenopausal women (Cundy et al. 1991; Mishell et al. 1972). However, given that amenorrheic athletes with estrogen levels between 30 pg/ml and 40 pg/ml (levels comparable to those observed among DMPA users), have lower lumbar bone densities than eumenorrheic athletes (Marcus et al. 1985; Snead et al. 1992), more research on the long-term impact of DMPA on bone integrity is warranted.
A subsequent follow-up report from New Zealand suggests that the bone-density loss among DMPA users is reversed after discontinuation of the method (Cundy 1994). A recent study in Thailand found no measurable differences in bone density between 50 DMPA users (who had been using the method for at least 3 years) and 50 IUD users (Taneepanichskul 1997). Measuring bone density at the distal and ultradistal forearm by dual energy X-ray absorptiometry (DEXA), the authors found mean bone mineral densities at the distal forearm were 0.48±0.05 g/cm2 in both groups, while bone mineral densities at the ultradistal forearm were 0.38±0.06 g/cm2 among DMPA users, and 0.4±0.05 g/cm2 among IUD users. These recent data are reassuring, but long-term prospective data in different populations are needed to characterize the impact of DMPA on bone densities.
No data are currently available on the possible impact of monthly, combined injectables on bone mineral density.
DMPA Effects on Vaginal Transmission of HIV
A study in 1996 reported that rhesus monkeys given subcutaneous progesterone implants, and subsequently exposed vaginally to the simian immunodeficiency virus (SIV), showed enhanced vaginal transmission of SIV, 7.7-fold over placebo implants (Marx et a1.1996). Progesterone treatment thinned the vaginal mucosal epithelium, a plausible mechanism for the observed effect.
The implications of this observation for humans are not clear. Available epidemiological data have largely suggested no measurable association between DMPA use and risk of becoming HIV infected. Ever use of DMPA bore no association to HIV infection in studies from Rwanda (Allen 1991), Tanzania (Kapiga 1994) or Kenya (Mati 1995). Two studies of current use of DMPA bore no association to HIV infection in Kenya (Mati 1995) or Thailand (Siraprapasiri 1991), yet a third study in Thailand reported a risk associated with DMPA (Rehle 1992). A prospective follow-up to the Rehle study reported a persistent increased risk of HIV sero-conversion among prostitutes using DMPA, compared with no method, or other methods (1995). A study carried out in Brazil on 20 DMPA users and 20 controls concluded that use of DMPA for 2-3 years did not affect vaginal epithelium thickness, Langerhans cell count or maturation index (Bahamondes et al. 2000).
There is no available data on the association between NET-EN and risk of HIV infection. The WHO and United Nations Programme on AIDS (UNAIDs) continue to recommend that women who believe themselves at risk of HIV infection should be encouraged to use female or male condoms; further studies on possible interaction of progestin-only contraceptives and HIV transmission are ongoing.
Due to the fact that combined, monthly injectable contraceptives contain estrogens, they can be expected to cause no thinning of the vaginal mucosa. However, there are no available epidemiological data on the association of combined, monthly injectables and HIV transmission.
Injectable Contraceptives under Development
Steroidal Preparations
A number of approaches are being used in the development of new injectable formulations. These aim at controlling the release of the active compound in order to avoid the peak serum level which follows the injection, and to extend the duration of action. Such improved pharmacokinetic profile would, in principle, allow a lower dose to provide the same efficacy, while minimizing the side effects of the contraceptive preparation.
Monolithic microspheres
Over the past few years, the Instituto Nacional de la Nutricion Salvador Zubiran and Applicaciones Farmaceuticas, both in Mexico, have developed a new method of synthesizing microspheres of steroids. The method uses a pulsating jet of melted drug to form microspheres, which are then annealed and sieved to obtain the desired range of particle size. These are then formulated to provide stable and long-lasting aqueous suspensions.
This technology is being explored for the development of testosterone microspheres which would have 60-90 days duration of action and could be used as androgen replacement or for male contraception. The technology is also being investigated with progesterone in order to develop a two-monthly injectable contraceptive for lactating women.
The most advanced preparation based on this technology is a combined injectable contraceptive containing 250 mg of progesterone and 5 mg of estradiol. It was tested in 30 women for a single cycle and appeared to block ovulation for a month. Work is continuing on reproducibility of the microspheres, and on the evaluation of the impact of multiple injections on vaginal bleeding patterns. This method is being extended to mixtures of natural steroids and cholesterol, as preliminary data suggest that such microspheres provide an even more favorable pharmacokinetic profile. Finally, the application of this technology to synthetic steroids such as levonorgestrel cholesterol, MPA or nestorone is under investigation.
Steroid Esters
In the mid-1970s HRP/WHO, in collaboration with the National Institute of Child Health and Human Development (NICHD, USA), established a program for the synthesis of new long-acting progestogens and androgens (Crabbé 1983). The approach chosen was to develop esters of known steroids, which would act as prodrugs and be converted to an active contraceptive agent by enzymatic hydrolysis in vivo.
Chemists from 12 countries worldwide synthesized 230 ester oximes and esters of norethisterone and levonorgestrel and 72 esters of testosterone. After purification and formulation, these compounds were tested in rodents, and in sub-human primates for the most promising ones. From these biological studies, it emerged that levonorgestrel esters were usually longer acting than the norethisterone esters; aqueous suspensions were generally better than oily solutions; and the duration of action of the longest acting agents was highly dependent on the crystal size of their aqueous suspensions.
After clinical testing, two compounds were selected for further development: levonorgestrel butanoate and testosterone buciclate. Levonorgestrel butanoate is being formulated as a three-monthly injectable contraceptive for women, and testosterone buciclate as a three-monthly injectable for male hormone-replacement therapy. The combination of both compounds is being considered as an injectable contraceptive for men, the inhibition of gonadotrophin secretion and suppression of spermatogenesis affected mainly by the progestogen, while the testosterone is provided primarily for androgen replacement.
Microsphere Formulations
The use of biodegradable poly-(lactide-co-glycolide) matrixes has been investigated for the delivery of natural and synthetic steroids as injectables. Investigators at Biotek, Inc. used this approach with progesterone, achieving sufficiently high loading of drug in the polymer to enable delivery of adequate amounts of progesterone for approximately 90 days in vitro and at least 77 days in rabbits. However, this work was stopped when safety issues were raised concerning the solvent used in the process. The same technology was investigated with testosterone and with synthetic steroids (norethisterone, levonorgestrel, norgestimate, nestorone), but these projects were abandoned because of additional concerns over reproducibility and cost.
Immunocontraceptives
Immunocontraceptives (Stevens et a1.1997) are based on the principle of using the body's own defense mechanisms to provide protection against pregnancy. Thus, they present a totally new approach to contraception. They have many potential advantages, including:
- Freedom from menstrual, systemic, metabolic and endocrine side effects
- Suitability for either men or women, depending on the target antigen
- No interference with sexual response or activity
- Sustained, reversible duration of action
- Use during all stages of a man's or woman's reproductive life
- Confidentiality of use
- Low manufacturing and storage costs
- Ease of distribution and administration within the health-care infrastructure.
A number of different targets are being used: hCG was chosen as the target for some formulations, because the hormone is usually only produced in women when fertilization and pregnancy occur, and it should not interfere with ovulation or the production of sex steroid hormones. Some of these preparations have reached the stage of clinical testing. Other immunocontraceptives are being developed that will exert their anti-fertility effect prior to fertilization. These preparations are intended for use by both men and women. They include immunocontraceptives directed against hypothalamic and pituitary hormones which are intended to inhibit ovulation and spermatogenesis, and immunocontraceptives directed against the mature ovum and spermatozoon which are intended to interfere with gamete interaction and fertilization. Some of the anti-hormone immunocontraceptives have reached the stage of initial assessment in clinical trials, but the anti-gamete immunocontraceptives are at an earlier stage of development.
hCG Immunocontraceptives
The most advanced immunocontraceptives are those based on hCG. Three main types have been developed to the stage of clinical testing:
- hCG beta subunit conjugated to tetanus toxoid ( hCG-TT)
- hCG beta subunit - ovine LH alpha subunit heterodimer conjugated to tetanus toxoid and diphtheria toxoid (HSD-TT-DT)
- hCG beta subunit C-terminal 37 residue synthetic peptide conjugated to diphtheria toxoid (CTP-DT).
All three preparations have been tested in phase-I (safety) clinical trials; they generated antibody levels estimated to provide protection against pregnancy in fertile women and no health hazards were noted. The HSD-TT-DT preparation was tested in a phaseII (efficacy) clinical trial and this study demonstrated that an hCG immunocontraceptive can be effective in preventing pregnancy.
However, none of these preparations will be suitable for general use. Their immunogenicity needs to be improved so that they can be effective in virtually all recipients, and their duration of action should be such that a single application can convey protection for at least 6 months. Thus, the objective of current research is to determine a more effective adjuvant than was used in the HSD immunogen already tested. The CTP-DT prototype was also abandoned, and a new product was developed that utilizes DT conjugates of two peptide segments of hCG: the original CTP portion (residues 109-145) and an analogue of a second peptide representing the amino acid 38-57 region. The resultant combined immunogen is more potent in inducing hCG reactive antibodies and these antibodies are much more effective in the bioneutralization of hCG in vitro and in vivo. In addition, biodegradable microsphere and emulsion technologies provide new opportunities for the sustained delivery of these preparations.
Sperm and Ovum Immunocontraceptives
Both sperm and ovum immunocontraceptives are currently being developed as methods to be used by women to prevent fertilization. Sperm antigens suitable for immunocontraception include sperm enzymes and sperm membrane glycoproteins.
The most promising enzyme is the sperm-specific isoenzyme of lactic dehydrogenase (LDH-C4) and preliminary studies in female baboons using recombinant and chemically-synthesized LDH-C4 peptide antigens have shown varying degrees of antifertility efficacy. A number of glycoproteins have also shown anti-fertility effects when used in in vitro systems or to immunize experimental animals. However, none of these antigens has reached the stage of clinical testing in humans.
The most well-studied ovum-associated antigens are the zona pellucida (ZP) glycoproteins. However, while animal studies confirmed that immunization with ZP proteins induced pronounced anti-fertility effects, this was accompanied by disturbances in ovarian function. Thus, current research aims at isolating specific peptide antigens, particularly from the primary sperm receptor ZP3, or to develop recombinant ZP proteins, to achieve a safe method. Another approach under investigation is the use of specific antigens in the oolemma, which may have the advantage of being restricted to the peri-ovulatory stage of oocyte development. In any event, the feasibility of developing a safe, effective and reversible ovum-based immunocontraceptive remains to be established.
Future Prospects
As a totally new approach to fertility regulation, immunocontraceptives will present new clinical challenges in order to ensure their safe delivery in family planning services, including monitoring the time to effect, maintenance of effect, and reversibility in individual users. Researchers are addressing these points by improving the formulations in order to reduce inter-individual variability, and by developing a means of inducing reversibility at will. The practicality of these methods remains to be confirmed.
Conclusion
Injectable contraceptives are a significant and growing share of worldwide contraceptive use. They offer many positive attributes, including a high measure of safety, high method effectiveness, the chance to avoid a daily pill, no interruption of coitus, different options for the length of effectiveness, and the chance for a woman to contracept without knowledge of her partner or family. For many women, these attributes are clearly very important, as indicated by the high method use worldwide.
At the same time, injectable methods have been a focus of concern by feminists for many years, due largely to the fact that these methods can be used covertly, and they cannot be discontinued or reversed until the drug has passed from the body. More recent concerns include the fact that these methods fail to protect women or men from sexually transmitted diseases, or the lethal threat of HIV.
The challenge for clinicians and family planning practitioners is to promote and prescribe contraceptive methods that are appropriate to the health risks, as well as the social needs and preferences, of the individual client. Current injectable methods offer safe and effective options for women not at risk of STDs or HIV; they can also provide an important component of dual protection for women wishing to combine a highly effective long-acting method with barrier protection.
An injectable mode of delivery for contraception has proven appeal for many women. Continuing research efforts to ensure the safety of available methods is very much warranted, particularly regarding the impact of progestogen-only methods on the risk of vaginal HIV transmission, and the long-term integrity of bone. Continuing research efforts to develop new, long-acting injectable contraceptives is to be encouraged if new products will offer measurable reductions in unwanted side-effects associated with current methods, particularly the disturbances to menstrual bleeding, and the delayed return of fertility.
References
- d'Arcangues C, Odlind V, Fraser I (1992) Dysfunctional uterine bleeding induced by exogenous hormones. In: N.J. Alexander, C. d'Arcangues (eds) Steroid hormones and uterine bleeding. Washington, AAAS Press, pp 81-105
- d’Arcangues C (2000) Management of vaginal bleeding irregularities induced by progestin-only contraceptives. Hum. Reprod. 15 [supp.3]:24-29
- Aedo AR, Landgren BM, Johannisson E et al. (1985) Pharmacokinetic and pharmacodynamic investigations with monthly injectable contraceptive preparation. Contraception 31:453-469
- Allen S, Lindan C, Serufilira A et al. (1991) Human immunodeficiency virus infection in urban Rwanda. JAMA 266:1657-63
- Amatayakul K, Sivassomboon B, Singkamani R (1980) Effects of medroxyprogesterone acetate on serum lipids, protein, glucose tolerance and liver function in Thai women. Contraception 21:283-298
- Ashman JAM (1990) Monthly injectable contraceptives and breast cancer (Master of Science thesis). University of Washington, WA, USA
- Bahamondes L, Lavin P, Ojeda G et al. (1997) Return of fertility after discontinuation of the one-a-month injectable contraceptive cyclofem. Contraception 55:307-310
- Bahamondes L, Diaz J, Petta C, Hall P (1998) Weight variation in users of the once-a-month injectable contraceptive Cyclofem. Adv. Contracept. 14:185-192
- Bahamondes L, Trevisan M, Andrade L, Marchi N, Castro S, Diaz J, Faundes A (2000) The effect upon the human vaginal histology of the long-term use of the injectable contraceptive Depo-Provera. Contraception 62:23-27
- Bledsoe CH, Hill AG, D'Alessandro P et al. (1994) Forthcoming. local cultural interpretations of western contraceptive technologies in rural Gambia. Population and Development Review, March
- Cookson KM (1994) The parenteral toxicity of Cyclofem. Contraception 49:335-345
- Coutinho E M, Spinola P, Barbosa I et al. (1997) Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate. Contraception 1997; 55:175-181
- Crabbé P, Archer S, Benagiano G, Diczfalusy E, Djerassi C, Fried J, Higuchi T (1983) Long-acting contraceptive agents: design of the WHO Chemical Synthesis Programme. Steroids 41:243-253
- Croxatto HB, Diaz S, Peralta 0 et al. (1983) Fertility regulation in nursing women: IV long-term influence of a low-dose combined oral contraceptive initiated at day 30 postpartum upon lactation and infant growth. Contraception 27:13-25
- Cundy, T et al. (1991) Bone density in women receiving depot medroxyprogesterone acetate for contraception. BMJ 303:13-16
- Cundy T, Cornish, J, Evans MC, Roberts H, Reid, IR (1994) Recovery of bone density in women who stop using medroxyprogesterone acetate. BMJ 308:247-248
- Cushman LF, Kalmuss D, Davidson AR et al. (1996) Beliefs about Depo-Provera among three groups of contraceptors. Adv Contracept 12:43-52
- Diaz S, Peralta 0, Juez G et al. (1983) Fertility regulation in nursing women: III. short-term influence of a low-dose combined oral contraceptive upon lactation and infant growth. Contraception 27:1-11
- Fotherby K, Benagiano G, Toppozada HK et al. (1982) A preliminary pharmacological trial of the monthly injectable contraceptive, Cycloprovera. Contraception 25:261-272
- Fotherby K, Saxena BN, Shrimanker K et al. (1980) A preliminary pharmacokinetic and pharmacodynamic evaluation of depot-medroxyprogesterone acetate and norethisterone oenanthate. Fertil Steril 34:131-139
- Fraser IS (1983) A survey of different approaches to management of menstrual disturbances in women using injectable contraceptives. Contraception 28:385-397
- Fraser IS (1994) Vaginal bleeding patterns in women using once-a-month injectable contraceptives. Contraception 49:399-420
- Fraser IS, Dennerstein GJ (1994) Depo-Provera use in an Australian metropolitan practice. Med J Aust 160:553-556
- Fraser IS, Diczfalusy E (1980) A perspective of steroidal contraception and abnormal bleeding: What are the prospects for improvement? In: Diczfalusy E, Fraser IS, Webb FTG (eds) Endometrial bleeding and steroidal contraception. Pitman Press Ltd, Bath, England, pp 384-413
- Garza-Flores J, Cardenas S, Rodriguez V, Cravioto MC, Diaz-Sanchez V, Perez-Palacios G (1985) Return to ovulation following the use of long-acting injectable contraceptives: a comparative study. Contraception 31:361-366
- Garza-Flores J, Guo-wei S, Hall P (1994) Population and delivery systems: variability in pharmacokinetics of long-acting injectable contraceptives. In: Snow R, Hall P (eds) Steroid contraceptives and women's response. Plenum Press, New York
- Garza-Flores J, Hall PE, Perez-Palacios G (1991) Long-acting hormonal contraceptives for women. J Steroid Biochem Mol Biol 40:697-704
- Guzman-Gracia S, Snow R, Aitken I (1997) Preferences for contraceptive attributes: voices of women in Ciudad Juarez, Mexico. Int Family Planning Perspect 23:52-58
- Hall PE (1987) Long-acting injectable formulations. In: Diczfalusy E, Bydgeman M (eds) Fertility regulation today and tomorrow - Raven Press, New York pp 119-141
- Hall PE, WHO Task Force (1994) The introduction of Cyclofem into national family planning programmes: experience from studies in Indonesia, Jamaica, Mexico, Thailand and Tunisia. Contraception 49:489-507
- Hassan EO, El-Nahal N, El-Hussein M (1994) Acceptability of the once-a-month injectable contraceptive Cyclofem and Mesigyna in Egypt. Contraception 40 (5): 469-488
- Hassan EO, El-Nahal N, El-Hussinie M (1999) Once-a-month injectable contraceptives, Cyclofem and Mesigyna, in Egypt. Efficacy, causes of discontinuation and side-effects. Contraception 60:87-92
- Herrero R, Brinton LA, Reeves WC et al. (1990) Injectable contraceptives and risk of invasive cervical cancer: evidence of an association. Int J Cancer 46:5-7
- Hickey M, d’Arcangues C (2001) Vaginal bleeding disturbances and implantable contraceptives. Contraception (in press)
- Howard G, Blair M, Fotherby K, Elder MG, Bye P (1985) Seven years clinical experience of the injectable contraceptive, norethisterone oenanthate. Br J Family Planning 11:9-16
- ICMR Task Force on Hormonal Contraception (1986) Return of fertility following discontinuation of an injectable contraceptive - norethisterone oenanthate (net en) 200 mg dose. Contraception 34:573-582
- Jaffe B, Harlap S, Baras M et al. (1988) Long-term effects of MPA on human progeny: intellectual development. Contraception 37:607-619
- Jaffe B, Shye D, Harlap S et al. (1989) Aggression, physical activity levels and sex role identity in teenagers exposed in utero to MPA. Contraception 40:351-363
- Jaffe B, Shye D, Harlap S et al. (1990) Health, growth and sexual development of teenagers exposed in utero to medroxyprogesterone acetate. Paediatr Perinat Epidemiol 4:184-195
- Johansson E, Odlind V (1987) The passage of exogenous hormones into breast milk - possible effects. Int J Gynaecol Obstet 25 [Suppl]:111-114
- Kapagi SH, Shao JF, Lwihula GK et al. (1994) Risk factors for HIV infection among women in Dar-es-Salaam, Tanzania. J Acquir Immune Defic Syndr 7:301-309
- Katz Z, Lancet M, Skornik J et al. (1985) Teratogenicity of progestogens given during the first trimester of pregnancy. Obstet Gynecol 65:775-780
- Kesserü EV, Aydinlik S, Etchepareborda JJ (1994) Multicentred, phase III clinical trial of norethisterone enanthate 50 mg plus estradiol valerate 5 mg as a monthly injectable contraceptive; final threeyear report. Contraception 50:329-337
- Koetsawang S (1994) Once-a-month injectable contraceptives: efficacy and reasons for discontinuation. Contraception 49:387-398
- Koetsawang S, Nukulkarn P, Fotherby K et al. (1982) Transfer of contraceptive steroids in milk of women using long-acting gestagens. Contraception 25:321-331
- La Veccia C (1994) Depot-medroxyprogesterone acetate, other injectable contraceptives, and cervical neoplasia. Contraception 49:223-230
- Lan PT, Aedo AR, Landgren BM, Johannisson E, Diczfalusy E (1984) Return of ovulation following a single injection of Depo-Medroxy-Progesterone acetate: a pharmacokinetic and pharmacodynamic study. Contraception 29:1-18
- Lei ZW, Wu SC, Garceau RJ et al. (1996) Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception 53:357-361
- Lumbiganon P (1994) Depot-medroxyprogesterone acetate (DMPA) and cancer of the endometrium and ovary. Contraception 49:203-209
- Marcus T. et al. (1985) Menstrual function and bone mass in elite women distance runners. Ann Intern Med 102:158-164
- Marx PA, Spira AI, Gettie A et al. (1996) Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med 2:1084-1089
- Mati JKG, Hunter DJ, Maggwa BN et al. (1995) Contraceptive use and the risk of HIV infection in Nairobi, Kenya. Int J Obstet Gynaecol 48:61-67
- McDaniel E, Pardthaisong, MA (1973) Depot-medroxyprogesterone acetate as a contraceptive agent: return of fertility after discontinuation of use. Contraception 8:407-414
- Mishell DR et al. (1972) Estrogenic activity in women receiving an injectable progestogen for contraception. Am J Obstet Gynecol 113:372-376
- Mukherjea M, Mukherjee P, Biswas R (1980) Long-term contraception with Depo-Provera: a clinical evaluation. Int J Fertil 25:122-126
- Newton JR, D'Arcangues C, Hall PE (1994) `Once-a-month' combined injectable contraceptives. J Obstet Gynaecol 14 [Suppl 1]
- Nutley T, Dunson TR (1997) Treatment of bleeding problems associated with progestin-only contraceptives: survey results. Adv. Contracept. 13:419-428
- Ortiz A, Hiroi M, Stanczyk FZ, Goebelsmann U, Mishell DR (1977) Serum medoxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of Depo-MPA. J Clin Endocrinol Metab 44:32-38
- Pardthaisong, T (1984) Return of fertility after use of the injectable contraceptive Depo-Provera: updated data analysis. J Biosoc Sci 16:23-24
- Pardthaisong T, Yenchit C, Gray R (1992) The long-term growth and development of children exposed to Depo-provera during pregnancy or lactation. Contraception 45:313-324
- Paul C, Skegg DCG, Spears GFS (1989) Depot medroxyprogesterone (Depo-Provera) and risk of breast cancer. BMJ 299:759-762
- Piya-Anant M, Koetsawang S, Patrasupapong N et al. (1998) Effectiveness of Cydofem in the treatment of DMPA-induced amenorrhea. Contraception 57:23-28
- Population Reports (1995) Series K, No.S, Baltimore, John Hopkins School of Public Health, Population Information Program
- Product Information (1994) Depo-Provera Contraceptive Injection. MI:Upjohn Company, Kalamazoo
- Rehle T, Brinkmann U, Siraprapasiri T et al. (1992) Risk factors of HIV-1 infection among female prostitutes in Khon Kaen, Northeast Thailand. Infection 20:328-331
- Sadana R, Snow R (1999) Balancing effectiveness, side-effects and work: women’s perceptions and experiences with modern contraceptive technology in Cambodia. Soc. Sci. Med. 49(3):343-58
- Sang GW, Shao QX, Ge RS et al. (1995) A multicentred phase III comparative clinical trial of Mesigyna, Cyclofem and injectable No.l given monthly by intramuscular injection to Chinese women. Contraception 51:167-183
- Saxena BN, Datey S, Gupta S et al. (1986) ICMR task force on hormonal contraception: return of fertility following discontinuation of an injectable contraceptive - norethisterone oenanthate (NET EN) 200 mg dose. Contraception 34:573-582
- Schwallie PC, Assenzo, Jr. (1973) Contraceptive use-efficacy study utilizing medroxyprogesterone acetate administered as an intramuscular injection once every 90 days. Fertil Steril 24:331-339
- Siegel I (1963) Conception control by long-acting progestogens: Preliminary report. Obstet Gynecol 21:666-668
- Siraprapasiri T, Rehle T, Brinkmann UK, Coplan P, Aiemsukawat C, Ungchusak K (1992) Risk factors of HIV- I infection among female prostitutes in Khon Kaen, Northeast Thailand. Infection. 20:328-331
- Skegg DCG, Noonan EA, Paul C et al. (1995) Depot medroxyprogesterone acetate and breast cancer. A pooled analysis of the World Health Organization and New Zealand Studies. JAMA 273:799-804
- Snead DB et al. (1992) Reproductive hormones and bone mineral density in women runners. J Appl Physio172:2149-2156
- Snow R, Garcia S, Kureshy N et al. (1997) Attributes of contraceptive technology: women's preferences in seven countries. In: Beyond acceptability: users’ perspectives on contraception. Reprod Health Matters pp 36-48
- Snow R, Hall P (eds.) (1994) Steroid contraceptives and women's response. Plenum Press, New York
- Stevens VC, Griffin PD, Jones WR (1997) Immunocontraceptives: an update. Bio Drugs
- Taneepanichskul S, Intaraprasert S, Theppisai U et al. (1997) Bone mineral density in long-term depot medroxyprogesterone acetate acceptors. Contraception 56:1-3
- Thomas DB, Molina R, Cuevas HR et al. (1989) Monthly injectable steroid contraceptives and cervical carcinoma. Am J Epidemiol 130:237-247
- Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T (1996) Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. J Acquir Immune Defic Syndr Hum Retrovirol 12:500-507
- UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. Annual Technical Report 1997
- UNDP/UNFPA/WHO/World Bank, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long-Acting Systemic Agents for Fertility Regulation (1997) Comparative Study of the effects of two once-a-month injectable steroidal contraceptives (Mesigyna and Cyclofem) on Lipid and Lipoprotein metabolism. Contraception 56:193-207
- UNDP/UNFPA/WHO/World Bank, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long-Acting Systemic Agents for Fertility Regulation 1998. Comparative Study of the effects of two once-a-month injectable steroidal contraceptives (Mesigyna and Cyclofem) on Glucose Metabolism and Liver Function. Contraception 57:71-81.
- Virutamasen P, Leepipatpaiboon S, Kriengsinyot R et al. (1996) Pharmacodynamic effects of depotmedroxyprogesterone acetate (DMPA) administered to lactating women on their male infants. Contraception 54:153-157
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Oral Contraceptives (1984) Effects of hormonal contraceptives on milk volume and infant growth. Contraception 30:505-522
- World Health Organization (1986) A multicentred phase III comparative clinical trial of depot-medroxyprogesterone acetate given three-monthly at doses of 100 mg or 150 mg: 1. Contraceptive efficacy and side effects. Contraception 34:223-236
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Long-acting Systemic Agents for Fertility Regulation (1987) A multicentred pharmacokinetic, pharmacodynamic study of once-a-month injectable contraceptives. I. Different doses of HRP112 and Depoprovera. Contraception 36:441-457
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Oral Contraceptives (1988a) Effects of hormonal contraceptives on breast milk composition and infant growth. Studies in Family Planning 19:361-369
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction. Task Force on Long-acting Systemic Agents for Fertility Regulation (1988b) A multicentred phase III comparative study of two hormonal contraceptive preparations given once-amonth by intra-muscular injection: I. Contraceptive efficacy and side-effects. Contraception 37:1-20
- World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives (1988c) Endometrial cancer and combined oral contraceptives. Int J Epidemiol 17:263-269
- World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives (1991a) Breast cancer and depot-medroxyprogesterone acetate: a multinational study. Lancet 338:833-838
- World Health Organization Collaborative Study of Neoplasia and Steroid Contraceptives (1991b) Depot-medroxyprogesterone acetate (DMPA) and risk of endometrial cancer. Int J Cancer 49:186-190
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long-Acting Systemic Agents for Fertility Regulation (1993) A Multicentre Comparative Study of Serum Lipids and Apolipoproteins in Longterm Users of DMPA and a Control Group of IUD Users. 47:177-191
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction. Task Force for Epidemiological Research on Reproductive Health (1994a) Progestogen-only contraceptives during lactation: I. Infant growth. Contraception 50:35-53
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction. Task Force for Epidemiological Research on Reproductive Health (1994b) Progestogen-only contraceptives during lactation: II. Infant development. Contraception 50:55-68
- World Health Organization, Family and Reproductive Health (2000) Improving access to quality care in family planning. Medical eligibility criteria for contraceptive use. Second edition [Doc. WHO/RHR/00.02]
- World Health Organization, Special Programme of Research, Development and Research Training in Human Reproduction, Task Force on Long-acting Systemic Agents for Fertility Regulation (1996) Clinical evaluation of the therapeutic effectiveness of ethinyl oestradiol and oestrone sulfate on prolonged bleeding in women using depot-medroxyprogesterone acetate (DMPA) for contraception. Human Reproduction 11 [suppl 2]:1-13
- Zhou X-F, Shao Q-X, Han X-J, Weng L-J, Sang G-W (1998) Pharmacokinetics of medroxyprogesterone acetate after single and multiple injection of Cyclofem® in Chinese women. Contraception 57:405-411