First Consensus Meeting on Menopause in the East Asian Region
Symptoms and treatment of the menopause: urogenital changes
Florante P. Gonzaga
Department of Obstetrics and Gynaecology, College of Medicine, University of the Philippines, Manila, Philippines
Introduction
Urogenital complaints such as urinary incontinence, dysuria, recurrent urinary tract infection, vaginal discomfort and dyspareunia have been reported by a large number of postmenopausal women. These symptoms cause considerable suffering and interference with their quality of life. The relationship between these complaints and oestrogen is well documented. Hormone replacement therapy (HRT) for the treatment of these complaints has benefited many of these women.
Urogenital complaints are one of the most common reasons for menopausal and postmenopausal women seeking consultation. There are no available figures for the whole of the Philippines; however, the most common complaints seen at the Philippine General Hospital Menopause Clinic are indicated in Table I.
Table I: Frequency of symptoms in women presenting at the Philippine General Hospital Menopause Clinic.
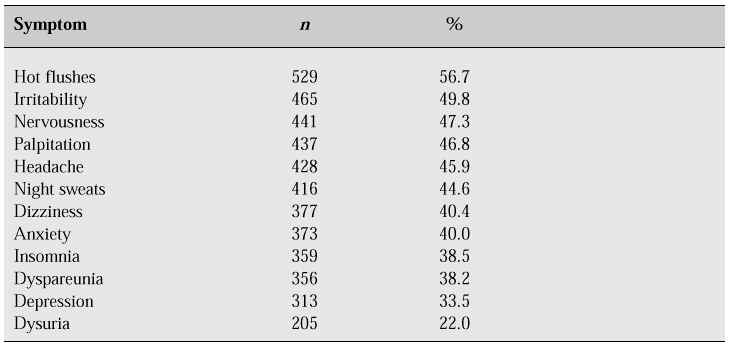
The incidence of degenerative and organic diseases of the urogenital system increases at the time of the menopause. Whether the hypo-oestrogenic state is the cause of the urogenital problems remains to be proven.
The true prevalence of postmenopausal urinary incontinence is unknown. There are no available data from the Philippines. In the West, different authors have reported prevalences varying from 14 to 49% depending on the definition, study design and geographical location [1–7]. In the menopause clinics, 20% of visiting patients complain of severe urgency and 50% of stress incontinence.
Epidemiology
The mean age of cessation of menses in the Philippines is 49 years, with an average life expectancy of about 25–30 years beyond the menopause. The projected life expectancy in the Philippines from the years 1900 to 2020 is shown in Table II.
Table II: Projected values of female life expectancy at birth in the Philippines, by region (1990–2020).1
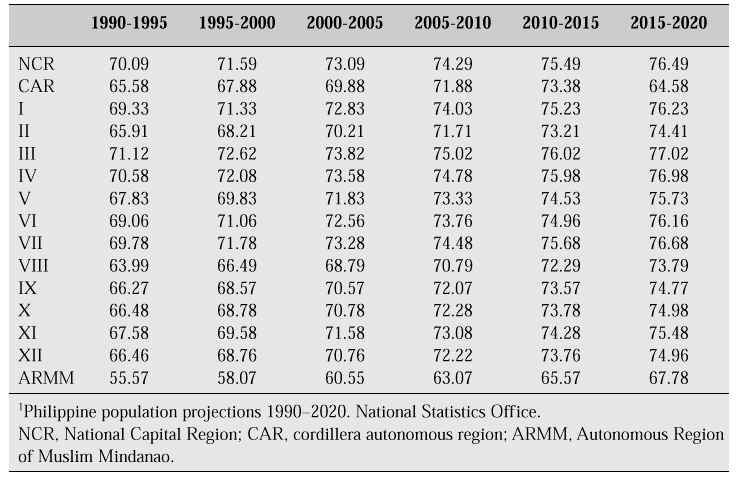
With increasing life expectancy we will see more women of menopausal age presenting with menopausal complaints.
Anatomy and physiology
The lower urinary tract
The genital and urinary tracts develop from a common embryological precursor — the urogenital sinus. The physiological effects of the menopause on the urinary tract are similar to those on the genital tract. After the menopause, the transitional epithelium of the trigone and the urethra becomes thinner. This is readily observed in the squamous epithelium of the trigone and the external urethral meatus. There is a diminished volume of blood flow through the urethral vascular plexus leading to a decrease in the normal coaptation of the urethral mucosa. Urethral support may be impaired due to a decrease in the elasticity of the connective tissue and atrophy of the striated muscle [8]. Low oestrogen levels lead to atrophic mucosal changes in the vagina, urethra and bladder, resulting in increased vaginal pH, altered vaginal flora and increased bacterial colonization with unfavourable flora such as Escherichia coli. Other factors such as loss of the glycosaminoglycan protective layer of the bladder, postvoid residual urine, impaired bladder emptying, increased faecal and urinary incontinence and increased bladder trabeculation and diverticula secondary to hypertrophy of the detrusor muscle contribute to increased susceptibility to bacterial infection.
Collagen constitutes approximately one-third of the total mass of the body and 25% of the total protein [9]. It is a major constituent of all connective tissue, 80% of which is in the dermis of the skin. There is increased crosslinking of collagen and a reduction in the total amount of collagen. The periurethral and urethral alpha-adrenergic receptors are reduced in number, resulting in a delay in conduction time.
The prevalence of symptomatic and asymptomatic urinary tract infection with age is probably due to low levels of oestrogen. Genitourinary atrophy leads to a great deal of discomfort. Urethritis with dysuria, urgency incontinence and urinary frequency are the result of mucosal thinning of the urethra and the bladder. The urethra and bladder are both affected by oestrogens.
Mechanism of continence
For urinary continence to exist, urethral pressure must exceed bladder pressure at all times except during micturition. The positive urethral closure pressure is produced by the four functional layers of the urethra — the epithelium, connective tissue, vascular tissue and muscle. All these layers are affected by oestrogen. Collagen is the most abundant structural protein. Collagen fibrils occupy twice the volume of muscle. Collagen is produced by fibroblasts which have oestrogen receptors [9]. In a study correlating skin collagen content with urethral function, increased collagen was associated with improved urethral sphincter function [10].
The function of the lower urinary tract is the storage and evacuation of urine. For normal function to occur, the bladder must fill with urine with minimal increases in intravesical (detrusor) pressure. This is known as accommodation. During the storage phase, bladder sensation should also be negligible such that one is not usually aware of the bladder filling. The bladder capacity must also be within a normal range for normal storage to occur and the reservoir function to be maintained. During the storage phase, the urethral sphincter must maintain a pressure (resistance) higher than the intravesical pressure and withstand a sudden increase in intraabdominal and intravesical pressure. Disorders of the storage phase lead to symptoms of urgency, frequency and urinary incontinence.
The evacuation phase depends on the ability to voluntarily initiate and sustain a detrusor contraction with simultaneous relaxation of the urethral sphincter. Disorders of the evacuation phase induce inadequate voiding leading to increased postvoid residual urine volume, decompensated detrusor function and upper urinary tract decompensation.
The urethra comprises the urethral sphincter, composed of mucosa, a vascular bed, connective tissue, and smooth and striated muscle fibres. Urethral pressure or resistance is derived from each of these anatomical components. The bladder and urethral sphincter are under the control of the central and peripheral nervous systems. The autonomic nervous system has both parasympathetic and sympathetic components. The parasympathetic system predominates over the bladder while the sympathetic system predominates over the urethral sphincter, particularly through alpha-adrenergic receptors located in the bladder neck. The central nervous system, in the form of a complex series of reflex arcs involving autonomic and somatic nerves, modulates bladder and urethral function. The coordination of these reflexes allows for efficient filling and emptying.
Oestrogen may aid continence by increasing urethral resistance, raising the sensory threshold of the bladder, increasing the alpha-receptor sensitivity in the urethral smooth muscle, or a combination of all the above. Oestrogen treatment has been shown to increase the number of intermediate and basal cells in the vagina of postmenopausal women, and similarly it has been observed in the urethra and bladder [11].
Types and mechanisms of urinary incontinence
Genuine stress incontinence represents the leakage of urine occurring during increases in intraabdominal pressure and is due to an inadequate sphincter mechanism. Inadequate sphincter is either due to a defective sphincter mechanism secondary to hypermobility of the urethra or due to inadequate intrinsic sphincter function. Atrophy can reduce urethral mobility in older women. Decreased periurethral tissue elasticity and density can decrease pressure transmission during exertion. Neuromuscular denervation, mucosal atrophy and muscular deterioration can add to genuine stress incontinence in older women.
Detrusor instability represents a type of incontinence due to involuntary contractions of the bladder which cannot be suppressed. This is idiopathic in 90% of women. Certain medical conditions such as cerebrovascular accidents, tumours, Parkinson’s disease and diabetes, uterine prolapse and chronic urinary tract infection may lead to detrusor instability.
Other physiological changes associated with ageing affect lower urinary tract function. These are conditions such as impaired cardiac function with congestive heart failure, venous stasis, oedema and nocturia. Neurological impairment such as peripheral neuropathy can lead to decreased awareness of bladder fullness or bladder overactivity. Diabetes may cause increased urine output with associated frequency of urination and nocturia. Decreased mobility can cause poor control of the lower urinary tract.
Vaginal relaxation with cystocele, rectocele and uterine prolapse, and vulvar dystrophies are not due to oestrogen deficiency. Although genuine stress incontinence is not affected by oestrogen treatment, there is an improvement in stress incontinence by 50% due to a direct effect on the urethral mucosa. In elderly women, urinary incontinence is usually a mixed problem with a significant component due to urge incontinence.
Urinary incontinence in postmenopausal women
Genuine stress incontinence is more common among women in their reproductive years when detrusor function is more operational in the bladder. It is important that adequate urodynamic investigation be carried out to evaluate the cause of the urinary incontinence.
Although symptoms of urinary incontinence have been associated clinically and temporally with the menopause, epidemiological and observational studies do not provide conclusive evidence that the overall prevalence of incontinence relates directly either to the menopause or to hypo-oestrogenaemia. There have been no well-controlled studies on the effect of oestrogen in women with incontinence, but there is evidence of symptomatic improvement.
Urethral pressure profilometry shows increased maximum urethral pressures and symptomatic improvement when conjugated oestrogens are given. Combined therapy using oestrogen and an alpha-adrenergic agonist produces better relief of symptoms of urinary frequency and micturition. Although oestrogen therapy alleviates symptoms of urgency, urge incontinence, frequency, nocturia and dysuria, there is no convincing evidence that oestrogen alone cures stress incontinence. A combination of oestrogen with an alpha-adrenergic agonist improves genuine stress incontinence [11–13].
Genital tract changes during the menopause
The genital tract undergoes anatomical and physiological changes after the menopause. There is a loss of vulvar subcutaneous tissue along with a thinning of the epidermis. The labia majora and minora decrease in size. The pubic hair density decreases and the vaginal epithelium becomes less cellular and thinner, leading to gradual loss of rugae and obliteration of the fornices. The vagina loses elasticity and becomes shorter, narrower and less distensible. The cellular glycogen diminishes, leading to a decreased colonization of Dšderlein’s bacilli and lactic acid production and an increased vaginal pH. This contributes to the increased prevalence of urinary tract infection and atrophic vaginitis.
Oestrogen receptors have been identified in the vagina, cervix, uterus, ovaries, fallopian tubes, urethra, trigone, bladder, connective tissues surrounding the urethra and muscles of the pelvic floor. There is vaginal dryness secondary to vaginal atrophy with a decrease in blood flow, decrease in vaginal fluid and reduced secretion during sexual stimulation. The vaginal changes can lead to vaginitis, pruritus, dyspareunia, stenosis or loss of libido after the menopause. With low oestrogen levels in the late menopausal years or years following oophorectomy, atrophy of the mucosal surfaces takes place. Postmenopausal bleeding may be due to atrophic vaginitis. There is, however, individual variability, and in some women urogenital atrophy develops shortly after cessation of menses, but in other women it develops several years later or even never.
Symptoms and diagnosis of genital tract atrophy
Many women with genital atrophy are asymptomatic while others suffer from pain, burning, dryness, discharge, dyspareunia, decreased vaginal lubrication, and vaginal bleeding. At the Philippine General Hospital Menopause Clinic, 38% of women complain of dyspareunia and 22% of dysuria.
Symptoms of urinary tract infection include frequency, urgency, dysuria, haematuria, and incontinence. The diagnosis can only be confirmed by either urinalysis and/or urine culture.
Diagnosis is usually made by evaluating the appearance of the vaginal mucosa. An atrophic mucosa is usually thin, pale, dry, without rugae, and the introital size is reduced. A Pap smear will confirm oestrogen deficiency and the parabasal cells will predominate, whilst the superficial cells will be minimal. The vaginal smear maturation value is a good measure.
Treatment
Lower urinary tract changesOestrogen therapy is a prophylactic treatment for recurrent urinary tract infections in the postmenopausal woman with urogenital atrophy. Chronic suppressive antibiotic therapy is frequently employed. Oestrogen therapy reverses mucosal atrophy, decreases vaginal pH and decreases colonization with pathogens.
Role of oestrogenThe sensitivity of the bladder and urethra to oestrogens has long been recognized. As early as 1941, Everett [14] postulated that the female urethra may be susceptible to atrophic senile urethritis compared with senile vaginitis as a result of oestrogen deprivation. There is a decrease in smooth muscle tissue in the lamina propria of the trigone and proximal urethra, as well as in the superficial muscular layer of the vagina, trigone and urethra in women at the time of the menopause. The decrease in urethral pressure after the menopause may be due to lack of oestrogen or to age-dependent changes such as decreased circulation, insufficient stimulation of alpha-adrenergic receptors and atrophy of soft tissue. Oestrogen receptors have been identified in the tissues of the urethra, bladder and pelvic floor musculature. Sensitivity and responsiveness to oestrogens have been noted in epithelial, connective, muscle, and vascular tissues. Urodynamic testing has shown that variations in oestrogen influence urethral vascular pulsations, urethral closure pressure and urethral pressure transmission. Our epidemiological data also show that lower urinary tract dysfunction is common after the menopause and that oestrogen replacement therapy can effectively treat this condition. Most cases can be improved by oestrogen therapy [15]. An immediate response is not expected. Usually a significant improvement occurs within 1 month, but it takes at least 6–12 months to fully restore the genitourinary tract.
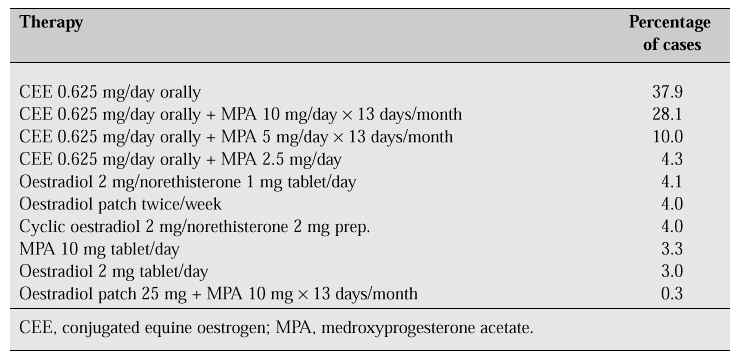
Genital tract atrophy responds to oestrogen administration. The different types of oestrogen and different routes of administration affect the therapeutic effect (Table III). Oral oestrogen is effective for the treatment of atrophy, whereas vaginal administration exerts a rapid local effect and avoids enterohepatic circulation. Oestrogen vaginal cream containing 0.625 mg conjugated oestrogen per gram can be given one to three times a day. Mandel et al. [16] showed that 0.3 mg/day vaginal conjugated oestrogen improves vaginal cytology similar to the effect of 1.25 mg oral conjugated oestrogen. The effect of vaginal conjugated oestrogen is fourfold greater than that of oral conjugated oestrogen in the vaginal epithelium. The duration of therapy shows that after 1 week, there is improvement in vaginal cytology, with a maximal response after 2 weeks [17]. A lower dose is enough to relieve vaginal atrophy, but a higher dose is necessary to relieve vasomotor symptoms. The lowest dose is always recommended for control of vaginal atrophy, but it may vary from patient to patient. The risk of developing endometrial carcinoma is very low and cyclic administration of progestogen and close follow-up of endometrial thickness using transvaginal ultrasonography are advised. A thickness above 5 mm requires endometrial sampling.
An alternative to oestrogen therapy is tamoxifen. Other unproven modalities of treatment include water-based jellies, creams and oils, vegetable oil, polycarbophil and yogurt. Continued sexual activity may prevent the development of vaginal atrophy by maintaining epithelial blood flow and pH in the normal range [18].
Table III: Frequency of prescribed regimens at the Philippine General Hospital Menopause Clinic.
Summary
Oestrogen receptors have been identified in the urethra, bladder and pelvic musculature. The epithelium, connective tissue, muscular and vascular tissue are responsive to oestrogen. Oestrogen can improve urethral vascular pulsations, urethral closure and urethral pressure transmission. Although these factors are necessary for urinary continence, genuine urinary stress incontinence can be due to other factors. Improvement in symptoms can be achieved with oestrogen therapy by as much as 50%. Recurrent urinary tract infections are improved with oestrogen therapy. Dyspareunia as a result of vaginal atrophy is markedly improved with HRT.
References
1. Milne JS, Williamson J, Maule MM. Urinary symptoms in older people. Mod Geriatr 1972; 2: 198.
2. Thomas TM, Plymat KR, Blannin J. Prevalence of urinary incontinence. Br Med J 1980; 281: 1243.
3. Yarnell JWG, St Leger AS. The prevalence and severity of urinary incontinence in women. J Epidemiol Commun Health 1981; 35: 71.
4. Vetter NJ, Jones DA, Victor CR. Urinary incontinence in the elderly at home. Lancet 1981; ii: 1275–7.
5. Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the non-institutionalized elderly. J Urol 1986; 136: 1022–5.
6. Iosif CS, Bekassy Z. Prevalence of genito-urinary symptoms in the later menopause. Acta Obstet Gynecol Scand 1984; 63: 257–60.
7. Molander U, Milsom I, Ekelund P, Mellstršm D. An epidemiological study of urinary incontinence and related urogenital symptoms in elderly women. Maturitas 1990; 12: 51–60.
8. Schaffer J, Fantl JA. Urogenital effects of the menopause. In: Barlow DH, ed. The menopause: key issues. Bailliere’s Clin Obstet Gynaecol 1996; 10/3: 401–17.
9. Iosif CS, Batra S, Ek A. Estrogen receptors in the human female lower urinary tract. Am J Obstet Gynecol 1981; 141: 817–20.
10. Versi E, Cardozo LD, Brincat M et al. Correlation of urethral physiology and skin collagen in post-menopausal women. Br J Obstet Gynaecol 1988; 95: 147–52.
11. Walter S, Kj¾gaard B, Lose G et al. Stress urinary incontinence in postmenopausal women treated with oral oestrogen (estriol) and alpha adrenoceptor-stimulating agent (phenolpropanolamine): a randomized double-blind placebo-controlled study. Int Urogynecol J 1990; 122: 74–9.
12. Beisland HO, Fossberg I, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females; treatment with phenylpropanolamine and estriol separately and in combination. Urol Int 1984; 39: 211–6.
13. Hilton P, Tweddell AL, Mayne C. Oral and intravaginal estrogens alone and in comination with alpha adrenergic stimulation in genuine stress incontinence. Int Urogynecol J 1990; 12: 80–6.
14. Everett HS. Urology in female. Am J Surg 1941; 52: 521–659.
15. Fantl JA, Wyman JF, Anderson RL et al. Postmenopausal urinary incontinence; comparison between non-estrogen-supplemented and estrogen-supplemented women. Obstet Gynecol 1988; 71/6: 823–8.
16. Mandel FP, Geola FL, Meldrum DR et al. Biological effects of various doses of vaginally administered conjugated equine estrogen in post menopausal women. J Clin Endocrinol Metab 1983; 57/1: 133–9.
17. Dyer GI, Young O, Townsend PT et al. Dose-related changes in vaginal cytology after topical conjugated equine estrogen. Br Med J 1982; 284: 789.
18. Leiblum S, Bachman G, Kemmann E et al. Vaginal atrophy in the postmenopausal woman. J Am Med Assoc 1983; 249: 2195–8.