Postgraduate Training Course in Reproductive Health/Chronic Disease
Incidence/prevalence of severe maternal morbidity - a literature review
Meile Minkauskiene, MD, PhD student
Clinic of Obstetrics and Gynaecology,
Kaunas University Hospital, Lithuania
Tutors: Lale Say, Ana Betran
World Health Organization
See also ![]() presentation
presentation
Abstract
Objective
- To summarise the prevalence/incidence of serious morbidity extracted from studies on severe maternal morbidity
- To compare studies designs and definitions
Methods
A systematic literature search revealed relevant studies reporting data on the prevalence/incidence of severe complications during pregnancy, delivery and postpartum period. For assessment of the quality of studies and for collecting the data, a structured data collection form from the WHO systematic review of maternal mortality and morbidity was used. Incidence/prevalence and case-fatality ratios were extracted.
Results
We included 38 reports, most of them were cross-sectional, hospital based (27/37) studies. Twenty two studies presented data about one severe condition (admissions to ICU, rupture of the uterus, hysterectomy, eclampsia, pre-eclampsia, acute abdomen, stroke, acute renal failure). Fourteen studies reported multiple causes of severe maternal morbidity (rupture of the uterus, haemorrhage, sepsis, eclampsia, dystocia, caesarean section, severe liver disorders) using different inclusion criteria depending on the severity of the disease.
Conclusions
The incidence/prevalence of severe maternal morbidity ranges from 0.07 to 8.23%, the case-fatality ratio from 0.02 to 37%. There is a big difference between case-fatality ratio in developing (South Africa 1:5; India and Niger 1:11) and developed countries (UK 1:118; France 1:222).
Background
The analysis of maternal deaths has long been used for the evaluation of women’s’ health and the quality of obstetric care. Around 10 to 20 maternal deaths per 100 000 live births are observed in countries in the European Union, less than one hundred deaths per year are registered in France (1) and so few cases exist now in England, Wales and Scotland, that the results are published every triennium for the whole United Kingdom (2).
Over the last decade the identification of cases of severe maternal morbidity has emerged as a promising complement or alternative to the investigation of maternal deaths (1). The studies revealed that near misses are a potentially useful starting point for audits. Scrutiny of near-misses may be useful for several reasons: larger numbers of cases could permit more contemporaneous reporting; lessons to be learned from the management of cases who survived may be at least as useful as from those who died; near-miss cases could also be seen as controls for deaths (1). If the requirement for total confidentiality is modified it may be possible to interview survivors and to help in determining both risk factors and substandard care. The ratio such as that of deaths to near-misses can be calculated and could be compared between regions or over time.
Although obstetric complications are sometimes presented as a relatively easy alternative to maternal deaths, difficulties remain in their definition and identification. A number of terms are in use to describe incidents of severe maternal ill health including life-threatening complications, severe maternal morbidity or near-misses.
Conceptually, morbidity during pregnancy represents part of a continuum between the extremes of good health and death. On this continuum, a pregnancy may be thought of as being uncomplicated, complicated, severely complicated or life threatening (Figure 1) (1). From these conditions, the woman may recover, she may be
temporarily or permanently disabled, or she may die.
Figure 1: Pregnancy continuum between extremes of good health and death.
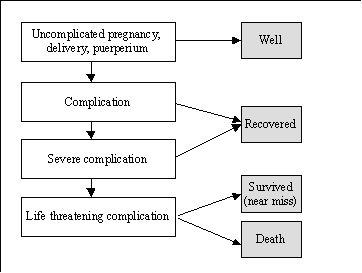
The main difficulty in designating where a woman is positioned on this continuum lies in the definition of the threshold of severity above which morbidity becomes severe or even life threatening. Whereas this threshold is easy to define for some conditions (e.g. few will disagree that a ruptured uterus is life threatening), it is not straightforward for others (e.g. what is the threshold for severe vaginal bleeding or for prolonged labour?).
An added difficulty is that the threshold above which an adverse obstetric event becomes life threatening may be context specific. The probability of a woman dying depends not only on the woman’s capacity to cope with a complication, but also on the access to and the quality of the care she receives. For example, a blood loss of 500 ml may not be life threatening in a non-anaemic healthy woman, but might put the mother’s life at risk if she is severely anaemic.
Finally, some diagnoses of severe obstetric complications may be particularly dependent on physician’s judgement. For example, cephalopelvic disproportion (CPD) in particular is difficult to ascertain. In the USA in the 1980s, there were six times more caesarean sections for CPD than in Ireland among women with a comparable risk status. But these differences were believed to reflect cultural factors rather than real differences in the epidemiology of CPD (1).
For all of these reasons, it is unlikely that standard, universally applicable definitions of severe morbidity can be proposed. The definitions obtained from the literature will need to be adapted according to the local context and the purposes of the investigation.
Three types of approaches have been proposed for defining life threatening obstetric complications and near miss events. These approaches include definitions based on (a) management, (b) clinical signs and symptoms, and (c) organ systems (1).
a. Management-based definitions
In developed countries, most of the definitions of life threatening obstetric complications and near miss events are management based. The management criterion most commonly used is admission to intensive care, regardless of the medical reason for the admission (3, 4, 5). The purpose of the majority of these investigations has been to describe the utilization patterns of obstetric care facilities or the use of general intensive care facilities for critically ill pregnant and postpartum women rather than to determine the rate of occurrence of life-threatening events related to pregnancy.
Other examples of management criteria used in the definition of life threatening complications include the use of emergency hysterectomy (6, 7), caesarean section (7), blood transfusion (7), hospitalization for more than four days (7), and anaesthetic accidents (6).
b. Definitions based on clinical signs and symptoms
Definitions based on clinical signs and symptoms are generally built around obstetric diagnoses or complications and tend to focus on the major causes of maternal mortality, such as haemorrhage, hypertensive disorders and sepsis. This approach is straightforward to interpret and has an immediate appeal for both clinicians and non-clinicians, particularly because the conditions listed tend to mirror the main causes of maternal death. In developing countries, data on broad diagnoses of complications might also be relatively easy to get from hospital registers.
The above points can be illustrated using the example of definitions for severe vaginal bleeding. In a European study, Bouvier-Colle and colleagues (8) based their definition of a life threatening postpartum haemorrhage on the following signs and symptoms: blood loss >= 1500 ml if measured, or haemorrhage leading to anomalies of coagulation. On the other hand, in a study in the UK, Stones et al (4) defined severe blood loss as that exceeding 2000 ml. In settings where the amount of blood loss is not routinely measured, other indicators of severe blood loss need to be sought. In Benin, for example, any postpartum haemorrhage associated with clinical signs of shock qualified as a near miss (9). Clearly, there appears to be no consensus as to what constitutes life threatening blood loss, and definitions will vary according to the context of the study.
c. Organ system-based definitions
Another approach to define near miss is that based on organ systems (6). A woman with organ failure or organ dysfunction (renal failure or cardiac decompensation, for example) during or within six weeks after pregnancy is very likely to die if she does not receive adequate care (6). For example, a haemorrhage might become life threatening if the bleeding leads to vascular (hypovolemia), renal (oliguria) or coagulation dysfunction. Infection, on the other hand, might become life threatening if the woman shows signs of respiratory (e.g. pulmonary oedema), immunological or cerebral dysfunction.
The actual frequency of severe maternal morbidity is not well known since there has been such scanty data of critical or serious illnesses during pregnancy and the puerperium. The purpose of this review is to summarise the prevalence/incidence of severe maternal morbidity from identified studies, to compare study designs and used definitions, and to discuss advantages and disadvantages of used methodologies.
Methods
Inclusion criteria
Studies which report data on prevalence/incidence of severe complications during pregnancy, delivery and postpartum are included. Studies with interventions for reduction of severe maternal morbidity were also included. Case-control studies or only case review studies without denominator were excluded.
Data collection
Medical databases, including Medline, Popline, Scielo from 1998 to 2003 were searched by key words "severe maternal morbidity" or "near-miss and maternal", limited to "human", "female and adults". The reference lists of identified articles were also reviewed. The following journals were hand searched: Lancet, European Journal of Obstetrics and Gynaecology, British Medical Journal, JAMVA. The ‘WHO systematic review of maternal morbidity and mortality database’ was scanned for studies from 1998 (67). Experts in the field were contacted. The title and abstract of the studies identified in the computerised search were scanned to exclude studies that were obviously irrelevant. The full texts of remaining studies were retrieved and scanned.
Quality of methods and data abstraction
For the assessment of the study quality structured data collection forms from the ‘WHO systematic review of maternal morbidity and mortality’ were used. The study quality was assessed by using the following criteria:
- description of study period
- information about population characteristics
- information about place of delivery
- description of the study settings
- information about eligible and lost subjects
- definitions of used conditions (morbidity or mortality)
- quality of forms of reporting data
- information about using special efforts to capture all maternal deaths
Data on the incidence or prevalence of severe maternal diseases and complications were extracted. The numbers and causes of maternal deaths were also collected. The case-fatality rate was calculated as the proportion of fatal cases among the reported cases of the specified diseases. The incidence rate for severe maternal morbidity is the ratio of cases with severe maternal morbidity at the beginning of a period per pregnant/post partum women-years at risk. The prevalence of near-miss cases is the ratio of cases with life-threatening maternal morbidity per total number of pregnant women (or live births) at a certain point of time.
Results
We identified 58 studies. Twenty studies were excluded: twelve of them did not have data on severe maternal morbidity prevalence or these data were not extractable (10-22), two were case-control design (23, 24), six - randomised placebo controlled trials (25-29). Therefore 38 studies were included in the review.
Baseline characteristics of the studies
Table 1 depicts the baseline characteristics of the included studies. Twenty seven studies were cross-sectional, and the prevalence/incidence and case-fatality ratios were calculated retrospectively by using hospital databases. Three studies were based on national data from the USA (39), Australia (41), Haiti (62), reporting different morbidity conditions. Only six studies are population-based: two of them have a cohort design (7, 49), one is a case-control study (56), four are prevalence/incidence surveys (58, 60, 61, 62). Hospital based were 25 studies, 4 of them were multicentric, 7 reported region/national data (7 studies). The sample size varied between 3777- 281 116.
Table 1. Characteristics of studies.
| Study | Country | Study design | Setting | Population | Sample size | Studied condition | Diagnosis | Comments |
| Al Sakka M 1998 (30) | Qatar | Cross-sectional | Hospital | Unknown | Rupture of uterus | Full thickness uterine wall defect | 65% hysterectomy | |
| Rajab SH 1998 (31) | Botswana | Cross-sectional | Hospital | Unknown | 21296 | Rupture of uterus | Diagnosed by laparotomy | 67% blood transfusion, 44% hysterectomy |
| Amata AO 1998 (32) | Nigeria | Cross-sectional | Hospital | Unknown | 4625 | Rupture of uterus | Diagnosed by laparotomy | 32% complicated by vesicovaginal fistula. 84% due to prolonged labour |
| Fawzi HW 1998 (33) | Sudan | Cross-sectional | Hospital | Unknown | 9111 | Rupture of uterus | Diagnosed by laparotomy | 50% hysterectomy |
| Bakour SH 1998 (34) | Syria | Cross-sectional | Hospital | Unknown | 31642 | Rupture of uterus | Diagnosed by laparotomy | 56% hysterectomy, 85% blood transfusion |
| Nasrat HA 1998 (35) | Saudi Arabia | Cross-sectional | Hospital | Rural | 18842 | Peripartum hysterectomy | 43.5%(10) due to uterine atony, 30.4%(7) - ruptured uterus, 26.1%(6) - placenta accreta | |
| Engelsen IB (36) 2001 | Norway | Cross-sectional | Hospital | Unknown | 70546 | Peripartum hysterectomy | Peripartum hysterectomy within 24h after delivery after 24th week of gestation | 7 cases due to uterine atony, 2 due to rupture of uterus |
| Nzerue CM 1998 (37) | USA | Cross-sectional | Hospital | Unknown | 70559 | Acute renal failure | Serum creatinin >71μmol/l | Acute renal failure causes: preeclampsia 8%, drug abuse 6%, HELLP – 50 % |
| Ali ME, 1998 (38) | Saudi Arabia | Cross-sectional | Hospital | Unknown | 15562 | Acute abdomen | Clinical symptoms | Causes: appendicitis (50%), cholecystitis (25%), ovarian cyste (10%) and others |
| Lanska DJ 1998 (39) | USA | Census | National | Mixed | 281116 | Stroke: subarachnoid and intracerebral haemorrhage, and ischemic stroke. Cerebral venous thrombosis and thrombosis of intracranial venous sinuses | According ICD-9-CM. | Sampling with stratification of hospitals by number of beds and geographic location |
| Tajammul A 1998 (40) | Pakistan | Cross-sectional | Hospital | Mixed, but most rural | Unclear | Eclampsia | Women presenting with convulsions in pregnancy or pregnant women who developed convulsions while admitted with a sBP>140mmHg or dBP>90mmHg or both | PM – 31.6% Death due to pulmonary oedema |
| Riley M, 1998 (41) | Australia | Census | Region | Unknown | Unclear | Eclampsia | According ICD-9-CM | The aim of study – compare the quality of two databases |
| Chinayon P 1998 (42) | Thailand | Cross-sectional | Tertiary Hospital | Unknown | 167200 | Eclampsia | Unclear | PM – 11.7 %( for weight>1kg). Maternal deaths due to intracerebral haemorrhage. The study try to evaluate the magnesium sulfate regimen too. |
| Makhseed M 1998 (43) | Saudi Arabia | Cross-sectional | Hospital | Unknown | 10407 | Pre-eclampsia | Used ACOG definitions | |
| Wacker J 1998 (44) | Zimbabwe | Cross-sectional | 2 Hospital | Mixed | 10750 | Pre-eclampsia | dBP>90mmHg and at least 1+ proteinuria dipstick | Try to examine the annual change in the incidence of preeclampsia |
| Tank PD 2002 (45) | India | Cross-sectional | Tertiary Hospital | Mixed | 6138 | Severe liver disease: HELLP,acute acute hepatitis, fatty live | Based on clinical symptoms and laboratory findings | All women with acute fatty liver and fulminant hepatitis died. 90,1% cases of all severe liver diseases – HELLP syndrome. PM – 62% |
| Mc Cord 2001(46) | India | Cohort | Region | rural | 2905 | Emergency obstetric care: obstructed labour, haemorrhage, eclampsia, infection. | Unclear | PM – 3,6%. |
| Gielchinsky Y 2002 (47) | Israel | Cross-sectional | Hospital | Unknown | 34450 | Placenta accreta | Based on clinical or histological criteria | The definition very wide: included sonographic diagnosed retained placental fragments or difficult manual removed placenta too. Rate of hysterectomies- 3.5% |
| 1999(48) | Scotland | Cross-sectional | Hospital | Unknown | No data | Primary postpartum haemorrhage | More than 1500ml | The audit for prevention and management emergencies in labour. |
| Prual A 2000 (49) | 6 countries of West Africa | Cohort population- based prospective |
Total population | Mixed | 20326 | Haemorrhage, dystocia, sepsis, rupture of uterus, severe liver disorders, eclampsia, sepsis, cesarean section, thrombo-embolism, vesicovaginal fistula | Different clinical diagnoses (haemorrhage with blood transfusion, sepsis – septicaemia, peritonitis or odorous vaginal discharge with hospitalisation and others) | |
| Baskett TF 1998 (5) | Canada | Cross-sectional | Hospital | Mixed | 76119 | All admissions to ICU | All admissions to ICU due to hypertensive disorders (25%), haemorrhage (22%), sepsis (15%), pulmonary embolism, acute fatty liver | |
| Loverro G 2001(50) | Italy | Cross-sectional | Hospital | Unknown | 23694 | Admissions to ICU | All admissions to ICU: worsening of pre-eclampsia (76%), severe bleeding (15%) and others | PM – 10.6% |
| Souza JPD, 2002 (51) | Brazil | Cross-sectional | Tertiary Hospital | Unknown | 28660 | Admissions to ICU | All admissions to ICU: hypertensive disorders (41%), haemorrhage (15%) and sepsis(13%) | Stillbirth – 19% |
| Murphy DJ 2002 (52) | UK | Cross-sectional | Hospital | Unknown | 51756 | Admissions to ICU | All admissions to ICU: haemorrhage (24%), thrombosis, hypertensive disorders (32%), sepsis, amniotic embolism, cardiac problems (24%) and others. | PM – 14% Near-miss maternal mortality: all women admitted for ICU in pregnancy or up 42 days post partum. |
| Bhuinneain MN 2001 (53) | Ireland | Cross-sectional | 2 medical facilities | Unknown | 67650 | Admissions to ICU | All admissions to ICU: haemorrhage (11%), thrombosis, hypertensive disorders (15%), sepsis (11%), cardiac problems (31%), acute fatty liver and others. | Marker of serious morbidity – the transfer of an obstetric patient to ICU. |
| Duffy S 2001 (54) | Ireland | Cross-sectional | Hospital | Unknown | 20800 | Admissions to ICU | All admissions to ICU: haemorrhage (26%), thrombosis, hypertensive disorders (48%), sepsis , cardiac problems. | |
| Prual A 1998 (55) | Niger | Cross-sectional | 6 medical facilities | Unknown | 4081 | Severe obstetric morbidity :dystocia, uterine rupture, preeclampsia, eclampsia, haemorrhage, puerperal sepsis, infectious diseases and others | Diagnosis based on usual clinical examination. Haemorrhage – hypovolemic shock requiring urgent blood transfusion |
Severe complications from 28th week of gestation to42nd day post partum that would have resulted in death of the mother or definite disabling sequelae without medical intervention. |
| Mantel GD 1998 (6) | South Africa | Cross-sectional prospective multicentre 2 year audit |
Hospital | Unknown | 13429 | Near-misses: vascular, cardiac, immunological, coagulation, renal, respiratory dysfunctions, all ICU admissions, emergency hysterectomies, anaesthetic accidents | Clear definitions of haemorrhage (hypovolemia requiring >5U blood), abortion complications, sepsis(septic abortion, chorioamnionitis, puerperal sepsis), pulmonary oedema and others | Classification based on organ dysfunction and management A near-miss describes a patient with an acute organ system dysfunction, which if not treated appropriately, could result in death. |
| Waterstone M 2001 (56) | UK | Population- based multicentre case-control prospective study |
25 hospitals | Mixed | 48865 | Severe obstetric morbidity: severe preeclampsia, eclampsia, HELLP syndrome, severe haemorrhage, severe sepsis, uterine rupture | Clear very strict clinical definitions based on laboratory findings. For severe haemorrhage - >1500ml or >4U of blood or fall in Hg>40g/l | Focuses on morbidity associated specifically with pregnancy and for which management usually involves maternity care professionals. |
| Bernis L 2000 (7) | Senegal | Population- based cohort |
2 regions | Urban | 3777 | Maternal morbidity: haemorrhage, hypertensive disorders, sepsis, obstructed labour, others | The definition of severe obstetric morbidity was based upon clinical diagnosis and medical or obstetrical interventions. | Compare outcomes and management in two different regions. |
| Filippi V 1998 (9) | Benin | Cross-sectional | Hospital | Unknown | 4291 | Near-misses: eclampsia, haemorrhage, dystocia, puerperal infections. | Not specified | |
| Khosla AH 2000 (57) | India | Cross-sectional | Hospital | Rural | 5124 | Near-misses: eclampsia, haemorrhage,, puerperal infections, ectopic pregnancy, abortion with uterine trauma, anaesthetic complications. | Not specified | |
| Canada 2000(58) | Canada | Incidence/ prevalence study |
National | Mixed | - | Severe maternal morbidity: amniotic fluid embolism, obstetrical pulmonary embolism, eclampsia, septic shock, cerebrovascular disorders, haemorrhage requiring transfusion or hysterectomy, catastrophic rupture of uterus, anaesthesia complications | Clear clinical diagnosis. Septic shock included with association to septic abortion, chorioamnionitis, pyelonephritis, endometritis . Rupture of uterus accompanied by bleeding | Data from different studies, prepared by Canadian Perinatal Surveillance System special group, using Discharge Abstract Database. Severe maternal morbidity – the number of women who experience severe (life-threatening) maternal morbidity per 100 000 live births in a given time and place. |
| Geller SE, 2002 (59) | USA | Cross-sectional | Hospital | Urban | - | Severe maternal morbidity and near-misses using classification by diseases (severe pre-eclampsia, eclampsia, embolism, infection...), morbid events (seizures, stroke, DIK, pulmonary oedema...), interventions (transfusion, ICU admission, hysterectomy...). | 164 women classified as having severe morbidity and 22 as near-miss morbidity using special qualitative assessment . | The aim of study- to study process for the definition and identification of near-miss morbidity with help of quantitative score. |
| Schoon MG 1999 (60) | South Africa | Incidence/ prevalence, population- based study |
Region | Mixed | 34100 | Vascular, cardiac, immunological, coagulation, renal, respiratory dysfunctions, all ICU admissions, emergency hysterectomies, anaesthetic accidents | Clear definitions of haemorrhage (hypovolemia requiring >5U blood), abortion complications, sepsis(septic abortion, chorioamnionitis, puerperal sepsis), pulmonary oedema and others | Near-miss – all cases with organ dysfunction or organ failure during pregnancy of any gestation until 42 days after termination of pregnancy. The study compare care in different regions and evaluate as optimal or suboptimal |
| Vandecruys HB 2002(61) | South Africa | Incidence/ prevalence multicentric population- based prospective study |
Region | Unknown | 40006 | Severe acute maternal morbidity: hypertensive disorders, pregnancy related and not related sepsis, haemorrhage, abortions complications, embolism and others. | Clinical. | Compare data of different years. |
| Haiti 1999(62) | Haiti | Incidence/ prevalence retrospective study |
National | Mixed | 80304 | Uterine rupture, dystocia, haemorrhage, eclampsia, fetal distress, hypertension, puerperal infection, ectopic pregnancy, breach presentation and many other | Not specified | Included all women who underwent a major obstetric intervention absolute maternal indication or died between 28th week of pregnancy and 42nd day postpartum. |
| Girard F 2001 (8) | France | Cross-sectional | Region | Unknown | 27875 | Severe maternal morbidity: severe haemorrhage, maternal sepsis, pregnancy induced hypertension. | Clear clinical definitions. Severe haemorrhage :>1500 ml or requiring blood and /or plasma expanders. Eclampsia – any fitting in pregnancy. |
Different definitions for the diseases were used. Five studies reported on obstetric patients treated in the intensive care unit (ICU) of tertiary hospitals. Other 18 studies also assessed only one specific condition or intervention (rupture of the uterus, hysterectomy, eclampsia, acute renal failure, acute abdomen, stroke, pre-eclampsia, severe liver disease, placenta accreta, primary postpartum haemorrhage). Fifteen studies used different morbidity definitions (near-miss, severe obstetric morbidity, severe maternal morbidity, acute severe maternal morbidity); all of them reported the prevalence of the disease. Most of the studies used conditions based on clinical signs and symptoms (8, 49, 56, 57, 61), few included organ systems based definitions (cardiac, immunological, renal dysfunction and others) (6, 60). The aim of Geller`s study (59) was to identify and classify all severe complications to near-miss morbidity or severe morbidity using special qualitative assessment. The differences of included conditions, used definitions, diagnostic evaluation of all studies are shown in table 1. The studies identified reported severe maternal morbidity only in the third trimester (from 22 or 28th week gestation) or all pregnancy diseases including peripartum and postpartum life-threatening conditions until the 42nd day after termination of pregnancy (table 2).
Table 2. Quality assessment of studies.
| Study | Location | Loss to follow-up (%) | Description of population characteri-stics | Data source | Description of study setting characteri-stics | Place of delivery | Forms of reporting data | Case definition | Maternal deaths definition | Comments |
| Al Sakka M, 1998 (30) | Qatar | 8.31% | yes | Medical records | no | Unknown | crude | clear | No deaths | |
| Rajab SH 1998 (31) | Botswana | - | no | unknown | 1 referral hospital | Mixed | crude | unclear | no | |
| Amata AO 1998 (32) | Nigeria | - | yes | Medical records | yes | Mixed | crude | unclear | no | |
| Fawzi HW 1998 (33) | Sudan | - | yes | Medical records | yes | Mixed | crude | unclear | no | |
| Bakour SH 1998 (34) | Syria | - | yes | Medical records | yes | Mixed | crude | unclear | no | |
| Nasrat HA 1998 (35) | Saudi Arabia | - | yes | Medical records | More high risk women | Unknown | crude | yes | no | |
| Engelsen IB 2001(36) | Norway | - | no | 4 different source: register of <Norway, delivery and operations register in hospitals, medical records | no | Unknown | crude | clear | no | |
| Nzerue CM 1998 (37) | USA | - | no | Medical records | Tertiary care hospital | Unknown | crude | clear | no | Included all cases of renal insufficiency (acute and chronic) |
| Ali ME 1998 (38) | Saudi Arabia | - | yes | Medical records of pregnant women, admitted to surgical ward | Tertiary care hospital | Unknown | crude | unclear | no | Not all women operated, could be wrong diagnosis |
| Lanska DJ 1998 (39) | USA | + | yes | Vital statistics | No settings | Hospital | Adjusted and standardized; used logistic regression | clear | no | Special efforts to capture for the multiple hospitalizations. Different identification of deliveries and hospitalizations due to the specific disease |
| Tajammul A 1998 (40) | Pakistan | - | yes | Unknown | yes | Mixed | crude | clear | no | Not clear method of collection data: prospective or retrospective, from medical records or from women |
| Riley M 1998 (41) | Australia | - | no | 2 different vital statistics databases | Clear description of both databases | Unknown | crude | clear | no | |
| Chinayon P 1998 42) | Thailand | - | no | Medical records | yes | Unknown | crude | No definition | no | |
| Makhseed M 1998 (43) | Saudi Arabia | 0.47% | no | Medical records | no | Unknown | crude | unclear | no | |
| Wacker J 1998 (44) | Zimbabwe | - | yes | Medical records | yes | Unknown | crude | clear | no data | |
| Tank PD 2002 (45) | India | - | no | Medical records | yes | Unknown | crude | clear | no | |
| Gielchinsky Y, 2002 (47) | Israel | - | no | Medical records | no | Unknown | Adjusted and standardised; used multivariate logistic regression | clear | no | |
| Scotland 1999(48) | Scotland | - | no | Audit (case review and others) | yes | + | Adjusted and standardised | yes | no deaths | |
| Prual A 2000 (49) | 6 countries in West Africa | 5.70% | yes | Multiple source: interview, clinical examinations, records | yes | mixed | Adjusted and standardized, used multivariate logistic regression | clear | yes | special effort to capture all deaths |
| Baskett TF 1998 (5) | Canada | - | no | Medical records | yes | Unknown | crude | no | no | |
| Loverro G 2001 (50) | Italy | - | no | Medical records | no | Unknown | crude | no | no | |
| Souza JPD 2002 (51) | Brazil | - | no | Medical records | yes | Unknown | crude | yes | no | |
| Murphy DJ 2002 (52) | UK | - | no | Multiple source: maternity database, ICU admissions database | yes | Hospital | crude | yes | no | |
| Bhuinneain MN 2001(53) | Ireland | - | no | Medical records | no | Unknown | crude | no | no | |
| Duffy S 2001(54) | Ireland | - | no | Medical records | yes | Unknown | crude | yes | yes | |
| Prual A 1998 (55) | Niger | - | yes | Multiple source: medical records and interview | no | Unknown | adjusted | no | no | Only definition of haemor-rhage |
| Mantel GD 1998 (6) | South Africa | - | no | Medical records and every morning audit meetings | yes | Unknown | crude | yes | yes | |
| Waterstone M 2001 (56) | UK | - | yes | Multiple source: medical records, staff reports, maternity computer database, labour ward diaries | yes | Unknown | adjusted and standardized, used multivariate logistic regression | yes | no | |
| Bernis L 2000 (7) | Senegal | 2.30% | yes | Multiple source: case records and interview | yes | Mixed | adjusted and standardized, used multivariate logistic regression | clear | yes | special effort to capture all deaths |
| Filippi V 1998(9) | Benin | - | no | Medical records | no | Unknown | crude | no | no | |
| Khosla AH 2000 (57) | India | - | rural | Multiple source: medical records and interview | no | Unknown | crude | no | no | |
| McCord C 2001(46) | India | 5.00% | rural | Multiple source: medical records and interview | yes | Mixed | adjusted and standardized, | yes | yes | |
| Canada 2000(58) | Canada | - | no | Vital | no | Unknown | - | yes | no | Data from different studies, prepared by Canadian Perinatal Surveillance System special group, using Discharge Abstract Database |
| Geller SE 2002 (59) | USA | 5.90% | urban | Multiple (hospital discharge financial database, hospital quality assurance reports, transport review logs, provider referral. Depart. Quality assurance reports). | yes | Unknown | crude | Special score for definition of severe morbidity or near-miss | no | |
| Schoon MG 1999 (60) | South Africa | 2.20% | mixed | Multiple: interview and case records | no | unknown | Standardised | yes | yes | |
| Vandecruys HB (62) | South Africa | - | yes | Multiple: daily audit meetings, case records | yes | unknown | Adjusted and standardised | yes | yes | |
| Haiti 1999(62) | Haiti | + | yes | Multiple interview and case records | yes | Mixed | Adjusted and standardised | unclear | yes | |
| Girard F 2001 (8) | France | - | yes | Medical records | yes | Unknown | adjusted and standardised, used multivariate logistic regression | yes | yes |
The more recent studies not only calculate incidence/prevalence data and try to reveal risk factors but also evaluate the quality of perinatal care. Several studies present morbidity data collected during audits in the hospital producing very clear conclusions about suboptimal or optimal care. A study in South Africa (61) compared incidence/prevalence ratio changes during several years when special measures to improve perinatal care and to reduce maternal mortality and morbidity were taken.
Quality assessment of studies
The quality assessment of the studies is presented in table 2. It shows that most of the studies are of poor methodological quality. Only 24% of studies present information about lost to follow-up cases, 45% of studies included a description of the studied population. Data sources varied: most of the studies used case records, 42% used multiple sources, but in two studies there was no information about it. Two studies used 4 or 5 sources to collect data: medical records, staff reports, maternity computer database, labour ward diaries, hospital discharge financial database, hospital quality assurance reports, transport review logs, provider referrals and others. Description about study settings was found not in all studies too (12 show as "-", there was not one word about setting characteristics). Some studies described the setting with only minimal characteristics, for example tertiary care or referral hospital. Information about the place of delivery is relatively rare and more often reported in studies from developing countries. Most studies had data about risk factors (66%) and more or less clear case definition (82%). Two studies used special efforts to capture all maternal deaths. Data reporting forms are usually crude, some recent studies report adjusted and standardised results.
Severe maternal morbidity ratios in studies
Table 3 shows the incidence/prevalence ratio and case – fatality. Large discrepancies exist between the surveys. Most of the variation in the rates and ratios described is due to different inclusion criteria. Many studies used deliveries as the denominator, making it easier to compare data. Canada presents the lowest morbidity ratio (less than 1). The other studies present similar incidence/prevalence ratios, varying between 1.1 – 8.23%. All four studies from South Africa report the highest case- fatality ratios: about 1 of 5 women with severe maternal morbidity died in this country. In India and Niger 1 out of 11 women with life-threatening complications died as compared to the United Kingdom with 1 of 118 or France with 1:222 maternal deaths are reported.. The very high case- fatality rate in South Africa may be due to different definitions or differences in perinatal care quality. The measured markers for severe maternal morbidity were different, and haemorrhage, sepsis, eclampsia are all included in all studies. Dystocia and cesarean section as a cause of severe morbidity reported only form studies conducted in developing countries, as they are less threatening conditions in developed countries. Haemorrhage as a marker was frequently used in studies from South Africa (49). Studies that used more strict severe haemorrhage criteria showed a prevalence ratio of less than 1%. For example, a population-based study from the United Kingdom (56) defining severe haemorrhage with a blood loss more than 1500 ml, reported a prevalence of 0.03%. The same criteria used in France by Girard (8) reported a ten times higher prevalence of haemorrhage - 0.39% and 3 times higher case-fatality ratio.
In Benin, Niger and India many deliveries are complicated by severe haemorrhage and 1 out of 11 women dies if she has this condition. The variations in sepsis prevalence are not so big and it may be due to a more clear definition and can be
explained only by the differences in perinatal care level. Case-fatality ratios in all countries are extremely different: few women die due to sepsis in Canada, Senegal, France, but in Niger and South Africa these numbers are between 50-72%. Half of the women with eclampsia died in India, but the average of case-fatality ratio in other studies due to eclampsia is 5%. The incidence of ruptured uterus is between 0.01-1%, and is associated with a higher mortality rate in some countries (South Africa). The prevalence of thrombo-embolism was reported only from Canada and South Africa, with high lethality in both countries: in Canada (depending on the treatment) 3-30%, in South Africa 83% -100%.
Table 3. The incidence/prevalence (1) (% ) and case-fatality ratio (2) (% ) of severe maternal morbidity studies.
| Study | Denominator | Severe maternal morbidity | Rupture of the uterus | Haemorrhage | Sepsis | Eclampsia | Dystocia | Cesarean section | Thrombo- embolism | Severe liver disorders |
|||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | ||
| Prual A 2000 (49), West Africa | Live births | 6.64 | 3.1 | 0.11 | 30 | 3.05 | 2.8 | 0.09 | 18 | 0.19 | 18 | 2.05 | 0 | 0.55 | 0 | 0.02 | 50 | 0.63 | 0 |
| Baskett TF 1998 (5), Canada | Deliveries | 0.07 | 3.6 | - | - | 0.02 | 8.3 | 0.01 | 0 | - | - | - | - | - | - | 0.01 | 25 | 0 | 0 |
| Prual A 1998 (55), Niger | Live births | 6.4 | 13.7 | 0.1 | 0 | 0.86 | 14,5 | 0.22 | 50 | 0.22 | 5.9 | 3.4 | 3.2 | - | - | - | - | - | - |
| Mantel GD 1998 (6), South Africa | Deliveries | 1.1 | 20.4 | - | - | 0.25 | 5.26 | 0.22 | 27.6 | - | - | - | - | - | - | - | - | - | - |
| Vandecruys HB 2002 South Africa (61) | 1.1 | 20 | - | - | 0.83 | - | 0.66 | - | - | - | - | - | - | - | - | 100 | - | - | |
| Waterstone M 2001 UK,(56) | Deliveries | 1.2 | 0.85 | 0.03 | 0 | 0.67 | 0.31 | 0.04 | 17.6 | 0.02 | 0 | - | - | - | - | - | - | 0.05 | 4 |
| Bernis L 2000, Senegal (7) | Deliveries | 7.1 | 5.4 | - | - | 2.25 | 6.02 | 0.22 | 0 | 1.68 | 4.8 | 2.2 | 0 | - | - | - | - | - | - |
| Filippi V 1998, Benin (9) | Deliveries | 8.23 | 8.5 | - | -- | 2.3 | 9.2 | 0.44 | 21 | 1 | 4.7 | 4.5 | 3.6 | - | - | - | - | - | - |
| Khosla AH 2000, India (57) | Deliveries | 4.37 | 13.84 | - | - | 1.31 | 9 | 0.74 | 26.3 | 1 | 52.4 | . | - | - | - | - | - | - | - |
| Mc Cord 2001, India (46) | Deliveries | 14.18 | 0.49 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Canada 2000, (58) | Births | - | - | 1 | low | >1, if vaginal delivery, 2-12 – if cesarean |
low | low | 3-50 | 0.05-0.2 | 5.8-14 | - | - | - | - | 0.3-0.001 | 3treated, 30 untrea-ted |
- | - |
| SChoon MG 1999, South Africa (60) | Deliveries | 5.3 | 37.4 | - | - | 0.06 | 27.3 | 0.03 | 72.7 | - | - | - | - | - | - | 0.02 | 83.3 | - | - |
| Haiti 2001 (62) | Deliveries | 2.9 | 0.02 | 0.01 | - | 0.52 | - | - | - | 0.2 | - | 0.01 | - | 1.12 | - | - | - | - | - |
| Girard F France, 2001 (8) | Births | 0.8 | 0.45 | - | - | 0.39 | 0.9 | 0.03 | 0 | 0..38 | 0 | - | - | - | - | - | - | - | - |
The results from studies about severe morbidity condition
Table 4 shows the definitions used for severe maternal morbidity. For many years ICU admissions used as indicators for a subset of parturient women at risk of severe morbidity. If we look at the structure of ICU according to disease it is seen that main causes are similar: haemorrhage, severe pre-eclampsia, sepsis, pulmonary embolism. But in intensive care we can see women with non-gynaecological diseases: injuries and accidents, surgical problems and others. The case-fatality ratio of women admitted to ICU is similar in all countries that show the importance of this serious morbidity marker: the indications to admit women to ICU or treat her in a hospital ward are not so different in different countries and hospitals showing that ICU admission ratios can be useful for comparisons between studies.
Table 4. The incidence/prevalence (%) and case-fatality ratio (%) according to severe maternal morbidity conditions.
| Study | Studied condition | Denominator | Prevalence/ incidence |
Case-fatality ratio |
| Al Sakka M 1998 (30), Quatar | Rupture of uterus | Delivery | 0.02 | 0 |
| Rajab SH 1998 (31), Botswana | Rupture of uterus | Delivery | 0.08 | 0 |
| Amata AO 1998 (32), Nigeria | Rupture of uterus | Delivery | 1.08 | 22 |
| Fawzi HW 1998 (33), Sudan | Rupture of uterus | Delivery | 0.15 | 7.14 |
| Bakour SH 1998 (34), Syria | Rupture of uterus | Delivery | 0.18 | 5.4 |
| Nasrat HA1998 (35), Saudi Arabia | Hysterectomy | Delivery | 0.12 | 4.45 |
| Engelsen IB 2001, (36)Norway | Hysterectomy | Delivery | 0.02 | 0 |
| Nzerue CM 1998 (37), USA | Acute renal failure | Women | 0.03 | 14.3 |
| Ali ME 1998 (38), Qatar | Acute abdomen | Delivery | 0.39 | 0 |
| Lanska DJ 1998 (139), USA | Stroke and cerebral venous thrombosis | Delivery | 0.03 | 2 |
| Tajammul A1998 (40), Pakistan | Eclampsia | Delivery | 0.75 | 5.26 |
| Riley M 1998 (41), Australia | Eclampsia | Delivery | 0.04 | No data |
| Chinayon P 1998 (42), Thailand | Eclampsia | Delivery | 0.05 | 3.3 |
| Makhseed M 1998 (43), Saudi Arabia | Preeclampsia | Delivery | 1.68 | No data |
| Wacker J 1998 (44), Zimbabwe | Preeclampsia | Delivery | 0.92 | No data |
| Tank PD 2002 (45), India | Severe liver disease | Delivery | 0.42 | 42.4 |
| Gielchinsky Y 2002 (47), Israel | Placenta accreta | Delivery | 0.9 | 0.32 |
| Scotland 2001 (48) | Postpartum haemorrhage | Delivery | 0.84 | 0 |
| Souza JPD 2002, (51) Brasil | Admissions to ICU | Delivery | 0.14 | 4.9 |
| Loverro G 2001, (50) Italy | Admissions to ICU | Delivery | 0.17 | 4.9 |
| Murphy DJ 2002 (52), UK | Admissions to ICU | Delivery | 0.1 | 6 |
| Bhuinneain MN 2001(53), Ireland | Admissions to ICU | Delivery | 0.04 | No data |
| Duffy S 2001 (54), Ireland | Admissions to ICU | Delivery | 0.09 | 5.26 |
The incidence of ruptured uterus in different counties large variation: in Quatar and Nicaragua the incidence is between 2-8:10000 and no maternal death , but in Nigeria one of 100 women has this complication during delivery and one out of five of them died. Hysterectomies in Norway are performed ten times less than in Saudi Arabia. For other conditions, such as preeclampsia, the ratio in two different countries may be very similar, but the eclampsia ratio can show a big difference (as shown in the previous table). Because preeclampsia incidence is independent from treatment or quality of care, the differences of eclampsia incidence could be useful for the evaluation of preeclamptic patient care. The case-fatality ratio for severe liver disease in India is very high when compared to that from the United Kingdom (56) and could be due to differences in treatment and pregnancy care in different countries.
Discussion
The results of analysis of these studies show that the incidence/prevalence ratio of severe maternal morbidity varies due to the different definitions, methods and classifications used. Ratios that are calculated by using different denominators, different diseases classifications with different degree of severity are not comparable between regions. Nevertheless, the reported incidence of severe maternal morbidity varies from 0.07—8.23% and lethality rates from 0.02-37%.
Maternal mortality risk is obviously dependant on the quality of care. There is a big difference between case-fatality ratio in developing (South Africa 1:5; India and Niger 1:11) and developed countries (UK 1:118; France 1:222). It is no secret, that appropriate care can decrease deaths due to severe maternal morbidity.
Severe maternal morbidity is easy to underestimate because pregnant women are usually healthy and recover quickly, and are discharged with relatively little follow-up. Some conditions might be life threatening in certain contexts while not in others, and different clinical criteria may need to be applied to define the life threatening nature of the complication (1). In all studies one or the combination of three types of definitions have been proposed for defining life threatening obstetric complications and near-miss events. These approaches include definitions based on (a) management, (b) clinical signs and symptoms, and (c) organ systems.
The most frequent used definition, based on management, was admission to ICU. The definition of a "near miss" may be identified by severity or by disease - in this case, admission to ICU was chosen as it was easy to measure. The main advantages of this definition are its simplicity and the ease of data collection since the use of only one register may be required. In addition, this definition encompasses non-obstetric medical conditions that might become life threatening and lead to death, for example, cerebral
haemorrhage and hepatitis. The major disadvantage is that intensive care may only identify a subset of life threatening conditions. In France, for example, most maternal deaths had been seen in intensive care units, while in Britain only a third of maternal deaths had received intensive care (1). Admission criteria to intensive care vary between countries, hospitals and clinicians, and the capacity and location of the intensive care unit also influences the number of admissions to these units (40). The definition of what constitutes an intensive care unit is in itself not clear and varies across hospitals (5), and some hospitals may not have an intensive care unit. Because of such variations, comparisons across settings have to be interpreted with caution.
Other examples of management criteria used in the definition of life threatening complications include the use of emergency hysterectomy (6, 7, 66), caesarean section (7,9), blood transfusion (7,9), hospitalization for more than four days (7), and anaesthetic accidents (6). In Benin for example, any woman with an obstetric haemorrhage necessitating a major intervention to stop the bleeding qualified as a near miss. Major interventions included hysterectomy, a blood transfusion of two litres or more for acute anaemia and coagulation defects, a manual revision of the uterus with extraction of placental fragments, a caesarean section, and a suture of the cervix or vagina (9). The main reason for this choice was that the interventions received were generally noted in the hospital records, while detailed clinical signs and symptoms were not. The above management criteria are prone to the same disadvantages as intensive care, however, since indications for their use may not be standardized and will differ between settings and clinicians (1). In South Africa for example, the definition of severe hypovolemia proposed for use in the tertiary hospital (i.e. hypovolemia requiring >= 5 units of whole blood
or packed cells for resuscitation) (6) had to be adapted for use in a rural district hospital. In the rural area, thresholds for transfusion were much higher and the volumes of blood given much lower. The definition for hypovolemia adopted was: if available, one would transfuse four units of blood or packed cells for resuscitation (these women should be included even if blood was not available) (1). The latter illustrates that where blood is not routinely available, relying on the need for transfusion will clearly not work, and other - probably clinical - criteria will have to be used.
Most of studies used definitions based on clinical signs and symptoms, such as haemorrhage, sepsis, hypertensive disorders, but this approach has several difficulties too. These definitions require the consensus of clinicians on criteria of severity, which can be difficult to obtain given the diversity of clinical experience. The criteria also depend on the means available to clinicians for making diagnoses. Finally, the criteria need to build on information that is routinely available in medical records to facilitate data extraction and verification (1).
The organ system-based definition, used in Mantel GD (6) and Schoon MG (60), is maybe the most accurate definition of a life threatening complication or a near miss in that only very severe endpoints are selected. This approach does not entirely avoid the weaknesses outlined above, in that the criteria used to define organ system failure or dysfunction may also rely on the management received (e.g. admission to intensive care or emergency hysterectomy) (1). In addition, the diagnosis of organ failure could require technologies which may not be available in many developing country hospitals (for example oxygen saturation).
Since the majority of cases of life threatening complications require hospital care to save the woman’s life, hospital records are the most likely source of information on these complications
(63). Although some have argued that cases can also be identified in the community because women can remember an event as distressing as a near miss, there is now substantial evidence that this is not the case. Studies assessing women’s recall of obstetric morbidity have found disagreement between the woman’s recall of her childbirth experience and medically diagnosed complications (63,64) or near miss. At a task force meeting on the validation of women’s reporting of obstetric complications, it was concluded that the estimation of the population prevalence of obstetric complications based on interview data collected in national surveys are not likely to be valid or reliable (65). While this does not mean that obtaining the community perspective on severe maternal illness is not possible, it does imply that life threatening adverse events need to be identified in the hospital first, or a study risks interviewing cases which are not truly life threatening (64).
The estimates of incidence probably underestimate the true incidence as case ascertainment is unlikely to be complete, especially if events occur outside the delivery suite and are not recognised; this may be particularly true of less serious cases. Only few studies used prospective data collection methods (6, 49, 56, 61). In countries with good access to health care hospital-based reviews can measure the incidence of serious conditions, because the untreated segment represents a smaller proportion of morbid population, but existing registers often need modification to supply the required data (64). Most hospital registers record the type of delivery and obstetric interventions, but specifying the complications as indications for the interventions appears to be inadequate. Within facilities, the data on obstetric complications often need to be collected from a series of registers and case notes, including admission, delivery, discharge, referral, intensive care and surgical registers.
(1). Geller (59) used five different data sources: 38% of cases were identified from hospital discharge financial database, 20% from hospital quality assurance reports, and another 20% from transport review logs. Obtaining information from several sources helps to achieve more exact results but only 50% of included studies in this review studies used this approach. Missing information seems to be more likely for emergency admissions, which are also likely to represent more serious complications. Women with complications are likely to be admitted in different wards of the hospital, and tracing patients across different registers is not easy because many registers lack a clear patient identification. Double counting may be a problem if more than one register needs to be consulted. Two studies conducted in the USA (39) and the UK (56) tried to solve this problem. If daily staff meetings are held, obstetric events can also be identified from a review of the case notes from these meetings (6, 61).
These dramatic differences are a good illustration of the problems when the studies are not population based. Measuring the morbidity in one or two departments in a teaching hospital or even in one or two clinics in a town, can lead to a biased picture of the real situation. Population based surveys (7, 49, 56, 60, 61) are preferable because the situation has to be depicted in a complete health area, taking into account all medical facilities playing a role in obstetric health fields.
Current data base and hospital records do not respond accurately to all purposes of measuring maternal morbidity. As there is no universal health indicator, it will be necessary to carry out specific surveys with the appropriate methodologies.
Conclusions
- Incidence of severe maternal morbidity ranges from 0.07—8.23%, case-fatality ratio 0.02-37%.
- There is big difference between case-fatality ratio in developing (south africa 1:5; india and niger 1:11) and developed countries (uk 1:118; france 1:222).
- Studies estimating the incidence of severe morbidity have used different definitions.
- Identifying cases of severe maternal morbidity requires sophisticated tools and clear definitions.
- Reviewing cases of severe maternal morbidity can provide useful complimentary insights into quality of care.
- A good quality medical system is required.
The severe maternal morbidity-mortality ratio can possibly be a new indicator of maternal care and could be used to compare improvements in treatments more accurately than mortality data alone. This major health risk to childbearing women is still relatively under investigated. Severe maternal morbidity is measurable and may be a more meaningful way to measure improvements in health care. Further work should identify those elements of the definition that are associated with poorer outcomes.
References
- Ronsmans C, Filippi V. Reviewing severe maternal morbidity: learning from women who survive life-threatening complications. In: Beyond the numbers. Geneva, WHO (in press).
- Department of Health, National Committee on Confidential Enquiries into maternal deaths. Why mothers Day, chapter 1, overreview,1998. London: HMSO.
- Lapinsky SE, Kruczynski K, Seaward GR, Farine D, Grossman RF. Critical care management of the obstetric patient. Can J Anaesth. 1997 Mar;44(3):325-9. [PubMed]
- Stones W, Lim W, Al-Azzawi F, Kelly M. An investigation of maternal morbidity with identification of life-threatening 'near miss' episodes. Health Trends. 1991;23(1):13-5. [PubMed]
- Baskett TF, Sternadel J. Maternal intensive care and near-miss mortality in obstetrics. Br J Obstet Gynaecol. 1998 Sep;105(9):981-4. [PubMed]
- Mantel GD, Buchmann E, Rees H, Pattinson RC. Severe acute maternal morbidity: a pilot study of a definition for a near-miss. Br J Obstet Gynaecol. 1998 Sep;105(9):985-90. [PubMed]
- de Bernis L, Dumont A, Bouillin D, Gueye A, Dompnier JP, Bouvier-Colle MH. Maternal morbidity and mortality in two different populations of Senegal: a prospective study (MOMA survey). BJOG. 2000 Jan;107(1):68-74. [PubMed]
- Girard F, Burlet G, Bayoumeu F, Fresson J, Bouvier-Colle MH, Boutroy JL. [Severe complications of pregnancy and delivery: the situation in Lorraine based on the European investigation] J Gynecol Obstet Biol Reprod (Paris). 2001 Oct;30(6 Suppl):S10-7. [PubMed]
- Filippi V, Alihonou E, Mukantaganda S, Graham WJ, Ronsmans C. Near misses: maternal morbidity and mortality. Lancet. 1998 Jan 10;351(9096):145-6. [PubMed]
- Walters WA, Ford JB, Sullivan EA, King JF. Maternal deaths in Australia. Med J Aust. 2002 May 6;176(9):413-4. [Free Full Text]
- Wagaarachchi PT, Graham WJ, Penney GC, McCaw-Binns A, Yeboah Antwi K, Hall MH. Holding up a mirror: changing obstetric practice through criterion-based clinical audit in developing countries. Int J Gynaecol Obstet. 2001 Aug;74(2):119-30; discussion 131. [PubMed]
- Lydakis C, Beevers DG, Beevers M, Lip GY. Obstetric and neonatal outcome following chronic hypertension in pregnancy among different ethnic groups. QJM. 1998 Dec;91(12):837-44. [Free Full Text]
- Kathula SK, Bolla SR, Magann EF. HELLP syndrome leading to a diagnosis of pregnancy. South Med J. 2002 Aug;95(8):934-5. [PubMed]
- Farina A, Barbieri M, Brown SA, Carinci P. Multivariate analysis for variables associated to preeclampsia. Am J Perinatol. 1998 Jan;15(1):1-2. [PubMed]
- Paarlberg KM, de Jong CL, van Geijn HP, van Kamp GJ, Heinen AG, Dekker GA. Vasoactive mediators in pregnancy-induced hypertensive disorders: a longitudinal study. Am J Obstet Gynecol. 1998 Dec;179(6 Pt 1):1559-64. [PubMed]
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol. 1998 Nov;105(11):1177-84. [PubMed]
- Moore JM, Nahlen BL, Lal AA, Udhayakumar V. Postpartum coma. Lancet. 1998 Aug 22;352(9128):658. [PubMed]
- Martey JO, Djan JO, Twum S, Browne EN, Opoku SA. Referrals for obstetrical complications from Ejisu district, Ghana. West Afr J Med. 1998 Apr-Jun;17(2):58-63. [PubMed]
- Supratikto G, Wirth ME, Achadi E, Cohen S, Ronsmans C. A district-based audit of the causes and circumstances of maternal deaths in South Kalimantan, Indonesia. Bull World Health Organ. 2002;80(3):228-34. [Free Full Text]
- Berg CJ, Bruce FC, Callaghan WM. From mortality to morbidity: the challenge of the twenty-first century. J Am Med Womens Assoc. 2002 Summer;57(3):173-4. [PubMed]
- Murray SF, Davies S, Phiri RK, Ahmed Y. Tools for monitoring the effectiveness of district maternity referral systems. Health Policy Plan. 2001 Dec;16(4):353-61. [PubMed]
- Begum MR, Akhter S, Begum A, Khatun M, Quadir E, Choudhury SB. Conservative management of eclampsia and severe pre-eclampsia--A Bangladesh experience. Medscape Womens Health. 2002 Jan;7(1):1. [Free Full Text]
- Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998 Dec;179(6 Pt 1):1539-44. [PubMed]
- Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, MacPherson C, Landon M, Miodovnik M, Paul R, Meis P, Dombrowski M. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998 Sep 3;339(10):667-71. [PubMed]
- Hofmeyr GJ, Nikodem VC, de Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. Br J Obstet Gynaecol. 1998 Sep;105(9):971-5. [PubMed]
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo-controlled trial. Am J Obstet Gynecol. 1998 Oct;179(4):1043-6. [PubMed]
- Golding J. A randomised trial of low dose aspirin for primiparae in pregnancy. The Jamaica Low Dose Aspirin Study Group. Br J Obstet Gynaecol. 1998 Mar;105(3):293-9. [PubMed]
- Rotchell YE, Cruickshank JK, Gay MP, Griffiths J, Stewart A, Farrell B, Ayers S, Hennis A, Grant A, Duley L, Collins R. Barbados Low Dose Aspirin Study in Pregnancy (BLASP): a randomised trial for the prevention of pre-eclampsia and its complications. Br J Obstet Gynaecol. 1998 Mar;105(3):286-92. [PubMed]
- Coetzee EJ, Dommisse J, Anthony J. A randomised controlled trial of intravenous magnesium sulphate versus placebo in the management of women with severe pre-eclampsia. Br J Obstet Gynaecol. 1998 Mar;105(3):300-3. [PubMed]
- Al Sakka M, Hamsho A, Khan L. Rupture of the pregnant uterus--a 21-year review. Int J Gynaecol Obstet. 1998 Nov;63(2):105-8. [PubMed]
- Rajab SH, Mulumba N. A 4-year clinical analysis of ruptured uterus. Int J Gynaecol Obstet. 1998 Dec;63(3):285-6. [PubMed]
- Amata AO. Anaesthetic and intensive care management of rupture of the gravid uterus: a review of 50 cases. Trop Doct. 1998 Oct;28(4):214-7. [PubMed]
- Fawzi HW, Kamil KK, Stronge J. Rupture of the uterus in labour: a review of 14 cases in general hospital. J of Obstetric and Gynecol 1998;18:429-430.
- Bakour SH, Nassif B, Nwosu EC. Outcome of ruptured uterus at University teaching Hospital Aleppo, Syria. J of Obstetric and Gynecol 1998;18:424-428.
- Nasrat HA, Youssef MH, Marzoogi A, Talab F. "Near miss" obstetric morbidity in an inner city hospital in Saudi Arabia. East Mediterr Health J. 1999 Jul;5(4):717-26. [PubMed]
- Engelsen IB, Albrechtsen S, Iversen OE. Peripartum hysterectomy-incidence and maternal morbidity. Acta Obstet Gynecol Scand. 2001 May;80(5):409-12. [PubMed]
- Acute renal failure in pregnancy: a review of clinical outcomes at an inner-city hospital from 1986-1996. J Natl Med Assoc. 1998 Aug;90(8):486-90. [PubMed]
- El-Amin Ali M, Yahia Al-Shehri M, Zaki ZM, Abu-Eshy S, Albar H, Sadik A. Acute abdomen in pregnancy. Int J Gynaecol Obstet. 1998 Jul;62(1):31-6. [PubMed]
- Lanska DJ, Kryscio RJ. Stroke and intracranial venous thrombosis during pregnancy and puerperium. Neurology. 1998 Dec;51(6):1622-8. [PubMed]
- Tajammul A, Saleem A, Aril A, Rasool N, Khan HT. Eclampsia. Pakistan`s J Med Sci 1998;14:111-118.
- Riley M, Halliday J. The accuracy of eclampsia cases reported to the Victorian Inpatient Minimum Database and the Perinatal Data Collection Unit. Health Inf Manag. 1998 Mar-May;28(1):13-5. [PubMed]
- Chinayon P. Clinical management and outcome of eclampsia at Rajavithi Hospital. J Med Assoc Thai. 1998 Aug;81(8):579-85. [PubMed]
- Makhseed M, Musini VM, Ahmed MA. Association of fetal gender with pregnancy-induced hypertension and pre-eclampsia. Int J Gynaecol Obstet. 1998 Oct;63(1):55-6. [PubMed]
- Wacker J, Schulz M, Fruhauf J, Chiwora FM, Solomayer E, Bastert G. Seasonal change in the incidence of preeclampsia in Zimbabwe. Acta Obstet Gynecol Scand. 1998 Aug;77(7):712-6. [PubMed]
- Tank PD, Nadanwar YS, Mayadeo NM. Outcome of pregnancy with severe liver disease. Int J Gynaecol Obstet. 2002 Jan;76(1):27-31. [PubMed]
- McCord C, Premkumar R, Arole S, Arole R. Efficient and effective emergency obstetric care in a rural Indian community where most deliveries are at home. Int J Gynaecol Obstet. 2001 Dec;75(3):297-307; discussion 308-9. [PubMed]
- Gielchinsky Y, Rojansky N, Fasouliotis SJ, Ezra Y. Placenta accreta--summary of 10 years: a survey of 310 cases. Placenta. 2002 Feb-Mar;23(2-3):210-4. [PubMed]
- Scottish audit of the prevention and management of emergencies in labour, an audit of progress safer childbirth. 2001, september. SPERCH.
- Prual A, Bouvier-Colle MH, de Bernis L, Breart G. Severe maternal morbidity from direct obstetric causes in West Africa: incidence and case fatality rates. Bull World Health Organ. 2000;78(5):593-602. [PubMed]
- Loverro G, Pansini V, Greco P, Vimercati A, Parisi AM, Selvaggi L. Indications and outcome for intensive care unit admission during puerperium. Arch Gynecol Obstet. 2001 Nov;265(4):195-8. [PubMed]
- Dias de Souza JP, Duarte G, Basile-Filho A. Near-miss maternal mortality in developing countries. Eur J Obstet Gynecol Reprod Biol. 2002 Aug 5;104(1):80. [PubMed]
- Murphy DJ, Charlett P. Cohort study of near-miss maternal mortality and subsequent reproductive outcome. Eur J Obstet Gynecol Reprod Biol. 2002 May 10;102(2):173-8. [PubMed]
- Ni Bhuinneain M, Barry-Kinsella C, Coughlan M, McKenna P, Bosio P. Critical care admission of obstetric patients. Ir Med J. 2001 Jan;94(1):26. [PubMed]
- Duffy S, Gaffney G. Maternal admissions to ICU--time to re-evaluate. Ir Med J. 2001 Sep;94(8):248-9. [PubMed]
- Prual A, Huguet D, Garbin O, Rabe G. Severe obstetric morbidity of the third trimester, delivery and early puerperium in Niamey (Niger). Afr J Reprod Health. 1998 Apr;2(1):10-9. [PubMed]
- Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case-control study. BMJ. 2001 May 5;322(7294):1089-93; discussion 1093-4. [Free Full Text]
- Khosla AH, Dahiya K, Sangwan K. Maternal mortality and 'near-miss' in rural north India. Int J Gynaecol Obstet. 2000 Feb;68(2):163-4. [PubMed]
- Perinatal Health Indicators for Canada: a resource manual. Severe maternal morbidity ratio. 2000, p.46-48.
- Geller SE, Rosenberg D, Cox SM, Kilpatrick S. Defining a conceptual framework for near-miss maternal morbidity. J Am Med Womens Assoc. 2002 Summer;57(3):135-9. [PubMed]
- Schoon MG. Analysis of all deaths in the province and all deaths and near-misses managed in health regions A and B. Report to the provincial head of health about maternal health study, 1999.
- Vandecruys HI, Pattinson RC, Macdonald AP, Mantel GD. Severe acute maternal morbidity and mortality in the Pretoria Academic Complex: changing patterns over 4 years. Eur J Obstet Gynecol Reprod Biol. 2002 Apr 10;102(1):6-10. [PubMed]
- UON network : Tackling unmet obstetric needs: Haiti.1999.
- Sadana R. Measuring reproductive health: review of community-based approaches to assessing morbidity. Bull World Health Organ. 2000;78(5):640-54. [PubMed]
- Fortney JA, Smith JB. Measuring maternal morbidity. Safe motherhood initiatives: critical issues. 1999, p.44-508. [Free Full Text]
- Bouvier-Colle MH. Frequency and characteristics of obstetrics patients admitted in intensive care unit: an example of severe reproductive morbidity in developed countries. Prepared for the Seminar on Innovative Approaches to the Assessment of Reproductive Health, organised by the Committee on Reproductive Health of the International Union for the Scientific Study of Population, 24-27 September 1996, Manila, The Philippines. [Free Full Text]
- Sahel A, Brouwere VD, Lardi M, Lerberghe WV, Ronsmans C, Filippi V. [Obstetric catastrophes barely just avoided: near misses in Moroccan hospitals] Sante. 2001 Oct-Dec;11(4):229-35. [PubMed]
- Systematic review on the epidemiological evidence for maternal morbidity and mortality 1997-2002. Department of Reproductive Health and Research, WHO, Geneva, 2002.